Abstract
Background:
Mixing of various adjuvants has been tried with local anesthetics in an attempt to prolong anesthesia from peripheral nerve blocks but have met with inconclusive success. More recent studies indicate that 8 mg dexamethasone added to perineural local anesthetic injections augment the duration of peripheral nerve block analgesia.
Aims:
Evaluating the hypothesis that adding dexamethasone to ropivacaine significantly prolongs the duration of analgesia in supraclavicular brachial plexus block compared with ropivacaine alone.
Patients and Methods:
It was a randomized, prospective, and double-blind clinical trial. Eighty patients of ASA I and II of either sex, aged 16-60 years, undergoing elective upper limb surgeries were equally divided into two groups and given supraclavicular nerve block. Group R patients (n = 40) received 30 ml of 0.5% ropivacaine with distilled water (2 ml)-control group whereas Group D patients (n = 40) received 30 ml of 0.5% ropivacaine with 8 mg dexamethasone (2 ml)-study group. The primary outcome was measured as duration of analgesia that was defined as the interval between the onset of sensory block and the first request for analgesia by the patient. The secondary outcome included maximum visual analogue scale (VAS), total analgesia consumption, surgeon satisfaction, and side effects.
Results:
Group R patients required first rescue analgesia earlier (557 ± 58.99 min) than those of Group D patients (1179.4 ± 108.60 min), which was found statistically significant in Group D (P < 0.000). The total dose of rescue analgesia was higher in Group R as compared to Group D, which was statistically significant (P < 0.00).
Conclusion:
Addition of dexamethasone (8 mg) to ropivacaine in supraclavicular brachial plexus approach significantly and safely prolongs motor blockade and postoperative analgesia (sensory) that lasted much longer than that produced by local anesthetic alone.
Keywords: Adjuvants, analgesia, dexamethasone, local anesthetics, peripheral nerve blocks
INTRODUCTION
Brachial plexus block is often used either as an adjuvant to general anesthesia (GA) or as a sole anesthesia modality. Brachial plexus blockade for ambulatory upper-limb surgery can significantly reduce pain and nausea, allowing for faster discharge from hospital when compared with GA.[1] This block remains the only practical alternative to GA for significant surgeries on upper limb. It provides a superior quality of analgesia and avoids the common side-effects associated with GA[2] and equally useful in patients with significant comorbidities.
Various local anesthetics alone or in combination with different adjuvants have been tried to prolong the duration of postoperative analgesia for a long time but have met with limited success. Bupivacaine is a well-established long-acting regional anesthetic, which like all amide anesthetics has been associated with cardiotoxicity when used in high concentration or when accidentally administered intravascularly. Hence, there is a need for a drug that can have all the advantages of bupivacaine without its cadiotoxicity. Ropivacaine is a long-acting regional anesthetic that is structurally related to bupivacaine. It is a S(−) enantiomer, unlike bupivacaine, which is a racemate, developed for the purpose of reducing potential toxicity and improving relative sensory and motor block profiles.[3]
However, still the challenge remains with anesthesiologists to prolong the duration of analgesia while minimizing adverse effects with single-shot brachial plexus block. Significant prolongation of brachial plexus analgesia is ideally accomplished with placement of continuous catheters. For moderate prolongation of analgesia (<24 h), various adjuvant drugs can be admixed with local anesthetic. There are no ultra long-acting local anesthetics or slow-release formulations clinically available.[4] Therefore, investigators have tried mixing local anesthetic with adjuvant drugs to prolong anesthesia from nerve block adjuvants including epinephrine, clonidine,[5,6] opioids,[7,8] ketamine,[9,10] and midazolam[11] have met with limited success. Corticosteroids have all been studied previously in an attempt to prolong the duration of analgesia after peripheral nerve blockade with varying degrees of success.[12,13] However, the glucocorticoid dexamethasone appears to be effective in a small number of preclinical[14,15] and clinical[16,17,18,19] studies. More recent studies indicate that 8 mg dexamethasone added to perineural local anesthetic injections augment the duration of peripheral nerve block analgesia.[17,20] Dexamethasone is very potent and highly selective glucocorticoid, its potency is about 40 times that of hydrocortisone. Clinical uses of dexamethasone are for treatment of many inflammatory and autoimmune condition.
The major goal in the management of postoperative pain is minimizing the dose of medication to lessen side effect while still providing adequate analgesia. Management of postoperative pain relieves sufferings and leads to early mobilization, shortened hospital stay, reduce hospital cost, and increase patient satisfaction. This technique of brachial plexus block seems to be a better alternative to GA in a new trend of day care surgeries.
We thus tested the hypothesis that adding dexamethasone to ropivacaine would significantly prolong the duration of analgesia in supraclavicular brachial plexus block compared with ropivacaine alone.
PATIENTS AND METHODS
Following Institutional Ethics Committee clearance as well as written informed consent, 80 patients (n = 80) of either sex aged between 16 and 60 years, ASA I and II undergoing elective upper limb surgery of not more than 2 h under supraclavicular brachial plexus block during a period of 2011-2013 were enrolled. It was a prospective, randomized, double-blind clinical trial. The patients were divided equally into two groups of 40 patients (n = 40) each using sealed opaque envelopes in the study as
-
(1)
Group R patients (n = 40) received 30 ml of 0.5% ropivacaine with distilled water (2 ml) → Control group
-
(2)
Group D patients (n = 40) received 30 ml of 0.5% ropivacaine with 8 mg dexamethasone (2ml) → Study group
Exclusion criteria are refusal to regional anesthesia, infection at site, local site anatomical abnormality, history of allergic reaction to study drugs, systemic use of corticosteroid for 2 weeks or longer, drug abuse, peripheral neuropathy, head injury, psychiatric disorder, severe pulmonary, cardiac, renal or endocrine disorder, peptic ulcer disease, pregnancy, and failure to achieve adequate block within 30 min of administration.
Aim and Objective
Primary outcome: The primary outcome was measured as duration of analgesia, defined as the interval between the onset of sensory block, and the initial use of rescue analgesia for surgical site pain.
-
Secondary outcome: They are as follows:
-
(1)To compare the effect of dexamethasone on block characteristic, that is, onset of block, peak effect of block, and duration of block
-
(2)To evaluate the efficacy of dexamethasone as an adjuvant with to 0.5% ropivacaine with plain ropivacaine in supraclavicular brachial plexus block in terms of maximum visual analogue scale (VAS) pain score and consumption of rescue analgesia
-
(3)Adverse effects such as nausea, vomiting, dysrhythmias, hypotension, convulsions, pneumothorax, pruritus, jerking movements, and hypersensitivity reaction for the study drug.
-
(1)
How to perform brachial plexus block
After getting clearance from preoperative assessment clinic (PAC), all patients included in the study were premedicated with tablet alprazolam 0.5 mg and ranitidine 150 mg orally at night before surgery and they were kept nil orally 12 pm onward. On arrival in the operating room, intravenous line was set using 18 gauge cannula and an infusion of Ringer's lactate was started as per calculation of perioperative fluid replacement therapy. The patients were monitored for heart rate (HR), noninvasive measurements of systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial blood pressure (MAP) at an interval of 5 min, continuous electrocardiographic (ECG) monitoring, and hemoglobin oxygen saturation (SPO2) throughout the perioperative period.
We used the classical approach to supraclavicular block using a single-injection with a nerve stimulator and a multiple stimulation technique for precise localization of each nerve in all the patients. All supraclavicular subclavian perivascular brachial plexus blocks were performed as described by Winnie using 22 G, a 50-mm insulated blunt needle (Stimuplex (B Braun)/8 Locoplex (Vygon) needles with extension tubing), and an Inmed nerve stimulator.
The intensity of stimulating current was initially set to deliver 1 mA with an impulse duration of 0.1 ms. Thereafter, current was gradually decreased to 0.5 mA or <0.5 mA until the proper motor response is achieved as described above. Following elicitation of an appropriate motor response with a current of 0.5 mA or <0.5 mA, 30 ml of the study drug was injected following repeated negative aspiration test in 3 ml incremental doses. Following injection, area was massaged so as to help the solution to track along the plexus. During the conduct of block and thereafter, the patient was observed vigilantly for any complications of the block and for the toxicity of the drugs injected. Immediately after block placement, patients were evaluated every 1 min up to 30 min for the onset of sensory and motor blockade, peak effect of sensory and motor blockade, quality of motor blockade, and also for total duration of sensory and motor blockade postoperatively. The above assessment was carried out by the principal investigator, who was blinded to the drugs administered in the plexus block. The study drug was prepared by an operation theatre technician not involved in the care or monitoring of the patients. The patients and the observing anesthesiologist and nurses were blinded to the study drug used. The following parameters were studied:
-
(1)
Onset of nerve blockade:
For sensory: Time from injection for brachial plexus block to reduction in sensibility to 30% or less (consider as onset of analgesia). Sensory block was assessed by pinprick using the blunt end of a 27-gauge needle comparing with contralateral arm.
For motor: Time from injection for brachial plexus block to reduction of muscle power equal to 3 (as per modified Lovett rating scale).
-
(2)
Complete (peak) nerve blockade:
For sensory: Time for injection for brachial plexus block to reduction in sensibility to 0 (pin prick test).
For motor: Time from injection for brachial plexus block to reduction of muscle power equal to 0 (as per modified Lovett rating scale).
-
(3)
Duration of analgesia:
The patients were asked to document the time when incisional discomfort began and the time when full power returned to the shoulder. Postoperative follow-up was carried out in the recovery and postoperative ward. The duration of analgesia was noted according to 0-10 VAS for pain at 0 h, every 1 h for first 3 h, and then 3 houly for next 24 h. When the patients began to experience the considerable pain and visual analogue scale measured VAS at 3-6, it was considered that analgesic action of the drug was terminated and rescue analgesic as tablet diclofenac 50 mg given. Even if patient complained of pain and visual analogue scale measured VAS > 6, rescue analgesic as injection diclofenac 75 mg was given.
-
(4)
Duration of motor block:
The duration of motor block postoperatively was assessed at 0 h, every 1 h for first 3 h and then 3 hourly for next 24 h by asking the patients to move their fingers and to see whether they are able to raise the hand or not. This time was recorded and taken as cessation of motor block effect.
-
(5)
Surgeon satisfaction:
Surgeons were interviewed at the end of study period to evaluate their views regarding nature of anesthesia and analgesia. We rated the satisfaction score as follows:
1 → satisfied; 2 → good; 3 → excellent.
-
(6)
Possible side effects of brachial plexus block:
Incidence of drowsiness, pruritus, nausea/vomiting, Horner's syndrome, phrenic nerve palsy, pneumothorax, respiratory depression, and signs and symptoms for local anesthetic toxicity were looked for and noted, if any.
Statistical analysis
Explanation if primary outcome is duration of analgesia: The sample size was calculated; taking standard deviation of 4 h based on the prior literature with a mean difference of 3 h between the samples. Assuming α-error (significance) of 0.05 and power (1- β) of 90%, the effective sample size on the basis of duration of analgesia came out to be 37 in each group for the comparison. Formula for calculating the sample size is as follows:
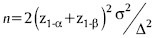
Explanation if primary outcome is VAS score: A sample size of 38 in each group will be sufficient to detect a clinically important difference of five points on the VAS of pain, assuming a standard deviation of 7.7 points, using a tow-tailed t-test of the difference between means, a power of 80%, and a significance level of 5%. The calculation is based on the assumption that the measurements on VAS scale are normally distributed. This number has been increased to 40 per group (a total of 80) to allow for a predicted dropout from treatment. Formula for calculating sample size is as follows:
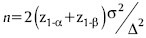
Baseline characteristics were compared using standard descriptive statistics. Visual analogue scale was presented as mean or median (interquartile range (IQR)) as required. The duration of analgesia was analysed by unpaired Student t-test. Categorical data were presented as percent of total. The results were considered significant if P value is <0.05 and highly significant if P value is < 0.001. All the statistical analysis was done with SPSS 16.0 version (Department of Preventive and Social Medicine).
RESULTS
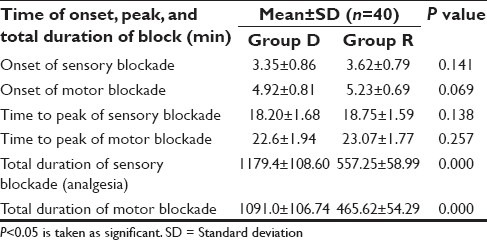
Eighty patients were enrolled and participated in the study. As per exclusion criteria, patients excluded from the study if they did not experience sensory block within 30 min of block administration. However, in our study, for the same reason, instead of excluding the patient from the study, we asked our OT technicians to allot that drug code to some other study patients to maintain study population size. Demographic characteristics and duration of surgery were comparable in both the study groups [Table 1]. The mean duration of onset of sensory and motor block and the mean duration of peak of sensory and peak of motor blockade were comparable in both the groups [Table 2].
Table 1.
Demographic characteristics of the two study groups
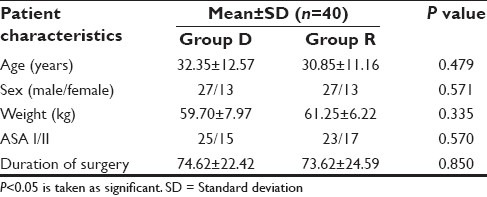
Table 2.
Onset and time to peak of sensory-motor blockade in two study groups
Duration of analgesia was taken as time from the onset of sensory blockade to the reappearance of pain. The mean duration of analgesia (sensory block) was longer in Group D as compared to group R (1179.4 ± 108.60 min versus 557.25 ± 58.99 min), which was highly statistically significant (P = 0.000) [Figure 1].
Figure 1.
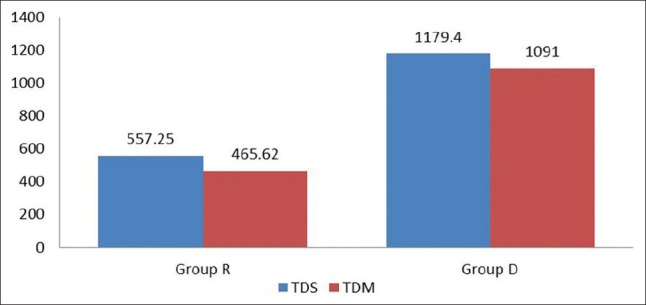
Total duration of sensory (analgesia) – motor blockade between two study groups
Visual analog scale for the monitoring and intervention of postoperative analgesia
VAS scoring was zero in both the study groups in the immediate postoperative period and remained as such up to almost 9 h of postoperative period and had no statistical significance. In Group R, patients required first rescue analgesia at 9 h 17 min (557 ± 58.99 min) while in Group D, patients required first rescue analgesia at 19 h 39 min (1179.4 ± 108.60 min), which was found statistically significant in Group D (P = 0.000) [Figures 2 and 3]. Group R patients received larger amount of first (98.75 mg in R versus 43.75 mg in Group D) as well a second rescue analgesia (75 mg in Group R versus 33.75 in Group D) compared to Group D patients, which was again statistically significant (P = 0.002 and 0.002, respectively).
Figure 2.
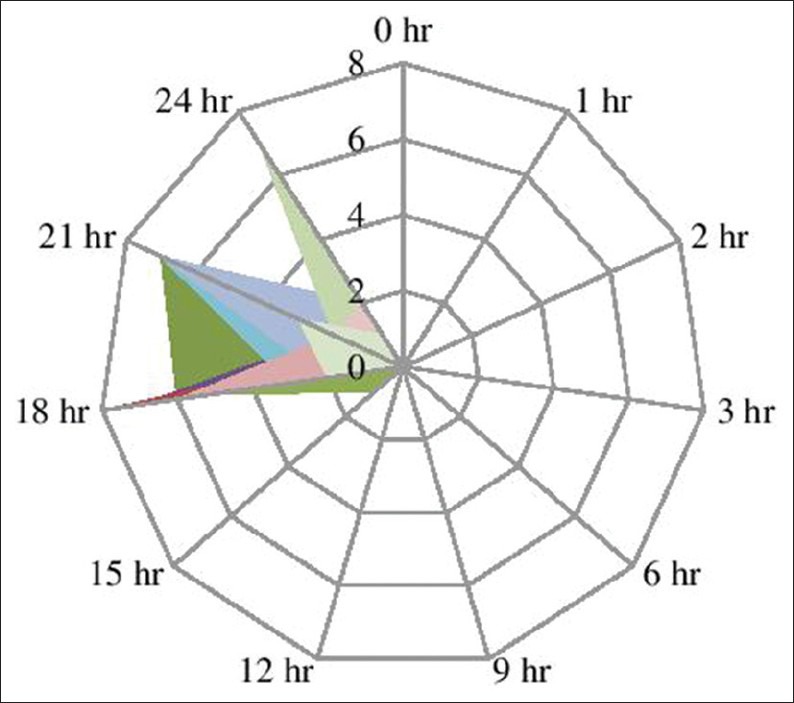
Visual analog scale for the monitoring and intervention of postoperative analgesia in group D patients
Figure 3.
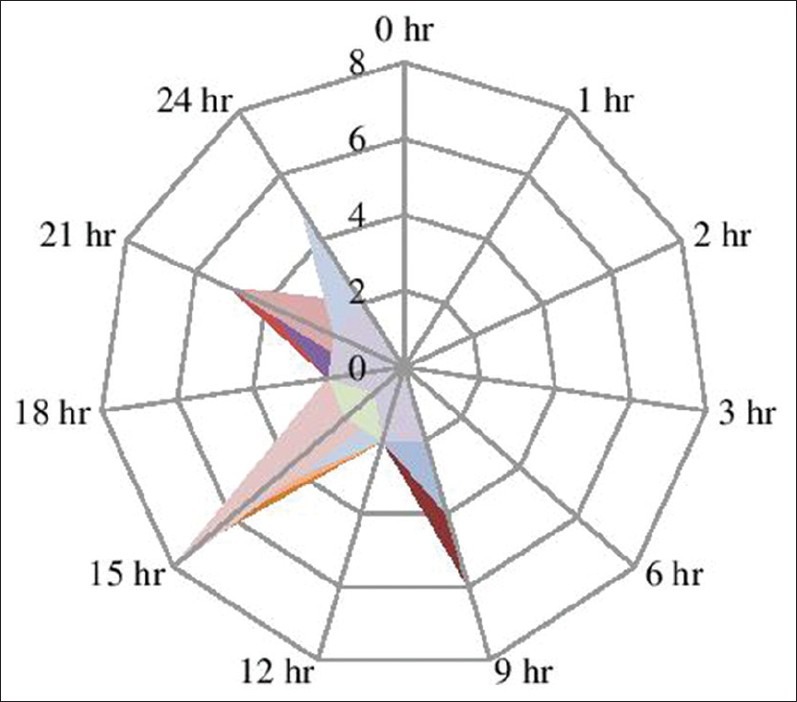
Visual analog scale for the monitoring and intervention of postoperative analgesia in group R patients
Surgeons’ satisfaction
We interviewed surgeons at the end of study period for anesthesia and analgesia using a rating scale ranging from 1 to 3. The satisfaction rate was higher in group D patients (excellent in 85% and good in 15%) than Group R patients (excellent in 62.5% and good in 37.5%).
Side effects
Neither a single patient complained of any side effects nor any sign of toxicity was visible in both the groups during the study period.
DISCUSSION
Supraclavicular block provides a rapid, dense, and predictable anesthesia of the entire upper extremity in the most consistent manner of any brachial plexus technique.[21] Although this is an excellent technique in experienced hands, the use of peripheral nerve stimulator has shown the considerable increase in the success rate of block and despite the advent of ultrasound-guided peripheral nerve blockade, nerve stimulation remains a popular technique used alone. Yasuda et al.[22] used a technique employing a nerve stimulator and an insulated needle for supraclavicular brachial plexus block that was successful in 98% of patients. That is why we had selected supraclavicular approach with peripheral nerve stimulator to supraclavicular brachial plexus block in our study.
Our study results demonstrate that the addition of dexamethasone to ropivacaine significantly prolongs the analgesic effect of plain ropivacaine postoperatively. These results are in keeping with the trend of previous studies using dexamethasone and a brachial plexus model;[16,18,19,23] however, accurate comparisons are challenging because of the variety of local anesthetic mixtures and adjuvants used, different blocks studied, and different methods of evaluating block duration. The block prolongation we observed (approximately twofold with ropivacaine and dexamethasone [Figure 1]; is consistent with observation of Pathak et al.,[21] who noticed significant prolongation of analgesia and motor block when dexamethasone was added to a mixture of 1.5% adrenalized xylocaine and 0.5% bupivacaine. However, Movafegh and colleagues[16] evaluated the effect of dexamethasone added to lidocaine using a nerve stimulator and found that the duration of surgery and the onset times of sensory and motor block were similar in both the groups, but the duration of sensory (242 ± 76 versus 98 ± 33 min) and motor (310 ± 81 versus 130 ± 31 min) blockade were significantly longer in the dexamethasone than in the control group (P < 0.01), which were similar to those of our study results [Table 2]. Vieira and colleagues[19] observed that adding dexamethasone to a mixture of bupivacaine, clonidine, and epinephrine increased interscalene block duration from 14 to 24 hours (1.7-fold prolongation). Their results, however, must be interpreted in the light of the presence of two α-agonists that were also included in the local anesthetic mixture. In contrast to previous studies in which dexamethasone was added to local anesthetic for brachial plexus block, we were unable to demonstrate the multifold augmentation of the duration of analgesia.[18,19,23] Differences in study methodology may have accounted for the observed differences in the duration of analgesia between studies, for example, the use of local anesthetics with longer duration of action,[18,19,23] larger volumes of injectate,[16,18,23] and the use of adjuncts such as epinephrine or bicarbonate with possible synergistic actions.[18,19,23] It is noteworthy that the addition of dexamethasone not only prolonged the duration of analgesia, but also may have contributed to an increased variability in the duration of analgesia.
Our study was different from other studies in that we designed it to assess a modest interaction between dexamethasone and the particular local anesthetic used. We opted to use ropivacaine without any other adjuvant, which would be less likely to mask any pharmacodynamic effect of adjunctive dexamethasone and found both statistically significant and clinically important interaction between these two.
Animal[24] and preliminary human studies[16,18,19,23,25] investigating the analgesic efficacy of dexamethasone added to local anesthetic agents have been found encouraging. It was selected in our study because, recently, dexamethasone has been studied as an adjuvant to local anesthetic in peripheral nerve block.[17,20] The mechanism of analgesia induced by corticosteroids is not fully understood. This effect is suspected to be mediated by their anti-inflammatory or immunesuppressive effects.[26,27] According to the traditional theory of steroid action, steroids bind to intracellular receptors and modulate nuclear transcription. Honorio et al.[28] found that steroids produce analgesia by blocking transmission in nociceptive c-fibers and suppressing ectopic neuronal discharge. According to Attardi et al.,[29] steroids might bring about this effect by altering the function of potassium channels in the excitable cells and this might be the probable mechanism of action by dexamethasone for the prolongation of blockade in our study. The dose of dexamethasone as an adjuvant to local anesthetics for peripheral nerve block has not been described. We selected a dose of 8 mg because administration of this dose seems to be safe in adults. Adverse effects with a single dose of dexamethasone are probably extremely rare and minor in nature, and the previous studies have demonstrated that short-term (<24 h) use of dexamethasone was safe.[30,31] Several studies have shown that addition of 4-8 mg of dexamethasone to local anesthetics effectively and significantly prolongs the duration of analgesia.[21]
Despite the concern surrounding the ‘off-label’ use of perineural adjuvants,[32] the safety profile of dexamethasone is promising. None of our study patient complained of neurotoxicity or any other complaints attributable to dexamethasone, although our sample size was insufficient to detect rare outcomes and we did not follow our patients beyond 24 h. Reports of corticosteroid-mediated neurotoxicity seem to be related to the vehicle polyethylene glycol and the preservative benzyl alcohol in steroid preparations as well as the presence of insoluble steroid particulate matter in the injectate,[33,34] neither of which applies to the formulation of dexamethasone (dexamethasone sodium phosphate) we used. The neurological risk, if any, of dexamethasone thus appears to be small. Although corticosteroids have been used successfully for postoperative pain relief in oral, general, and orthopaedic surgery,[35,36] other studies have not corroborated these reports.[30,37] Adding a steroid to local anesthetic solution may not be indicated for all patients. For example, diabetic patients may experience hyperglycemia and patients with a continuing infectious process may be detrimentally affected by the anti-inflammatory effects of steroids,[16] but concerns about steroid-induced hyperglycaemia have been borne out in high-dose I.V. regimens.[38] This led us to hypothesize that it may be useful in situations in which epinephrine must be used with caution (e.g. hypertension, ischemic heart disease).
In our study, group ‘D’ patients showed prolonged duration of sensory (557 ± 58.99 min in ‘R’ versus 1179.4 ± 108.60 min in ‘D’; P < 0.000) as well as motor blockade (456.62 ± 54.29 min in ‘R’ versus 1091 ± 106.74 min in ‘D’; P < 0.000), hence reducing the consumption of rescue analgesia in group ‘D’ patients. The amount of both, first and second rescue analgesia, was higher in group ‘R’ patients as compared to group ‘D’ patients (P < 0.00). None of our study patient required analgesia in the immediate postoperative period. As our postoperative follow-up period was limited to 24 h and hence the measurement of rescue analgesia also. While following 24 h onward, VAS scoring trend might have been different from day 1 scoring as tissue inflammation started to settle down.
Surgeons satisfaction score for surgical anesthesia and analgesia was found to be more excellent (85%) in group D than in group R (62.5%) patients, which indicates that surgeons were more satisfied in group D than group R. This may be explained in view of quality of supraclavicular block itself as it provides a rapid, dense and predictable anesthesia of the entire upper extremity in the most consistent manner of any brachial plexus technique. It consists of injecting local analgesic drugs in the fascial spaces surrounding the nerve plexus, thereby blocking the autonomic, sensory, and motor fibers supplying the upper extremity. It is carried out at the ‘division’ level of the brachial plexus, with high volume the ‘trunk’ level of the plexus may also be blocked in this approach.[39,40,41] Perhaps this is why there is often little or no sparing of peripheral nerve if an ‘adequate’ paraesthesia or stimulation is obtained. Second, a relative increase in the duration of analgesia was far more pronounced because of the use of long-acting local anesthetics, that is ropivacaine. Third, this is because of synergistic action of analgesic efficacy of dexamethasone added to local anesthetic agent. In conclusion, we found that addition of dexamethasone (8 mg) to ropivacaine (local anesthetic drug) as an adjuvant in supraclavicular brachial plexus significantly prolongs the duration of postoperative analgesia and motor block in patients undergoing upper limb surgeries which lasted much longer than that produced by local anesthetic alone. It was found to be well tolerated and cost-effective method without the occurrence of remarkable adverse effects. Although toxicity profile of dexamethasone appears to be well tolerated till date, but our study population size was small and the postoperative follow-up period was limited to 24 h only, to find out any conclusive complication. Therefore, further studies are required to evaluate the mechanism of action as well as the optimal dose before its routine use as a perineural adjunct can be advocated to prolong block characteristics.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.McCartney CJ, Brull R, Chan VW, Katz J, Abbas S, Graham B, et al. Early but no long-term benefit of regional compared with general anesthesia for ambulatory hand surgery. Anesthesiology. 2004;101:461–7. doi: 10.1097/00000542-200408000-00028. [DOI] [PubMed] [Google Scholar]
- 2.Kumar P, Raju BC, David CM. Ultrasound-guided brachial plexus block continuing education in anesthesia. [Last accessed on 2014 Mar 06];Critical Care and Pain Advence Access Published. 2013 Dec 11; [Google Scholar]
- 3.Hansen TG. Ropivacaine: A pharmacological review. Expert Rev Neurother. 2004;4:781–91. doi: 10.1586/14737175.4.5.781. [DOI] [PubMed] [Google Scholar]
- 4.Rose JS, Neal JM, Kopacz DJ. Extended-duration analgesia: Update on microspheres and liposomes. Reg Anesth Pain Med. 2005;30:275–85. doi: 10.1016/j.rapm.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Andan T, Elif AA, Ayse K, Gulnaz A. Clonidine as an adjuvant for lidocaine in axillary brachial plexus block in patients with chronic renal failure. Acta Anaesthesiol Scand. 2005;49:563–8. doi: 10.1111/j.1399-6576.2005.00631.x. [DOI] [PubMed] [Google Scholar]
- 6.Duma A, Urbanek B, Sitzwohl C, Zimpfer M, Kapral S. Clonidine as an adjuvant to local anaesthetic axillary brachial plexus block: A randomized, controlled study. Br J Anaesth. 2005;94:112–6. doi: 10.1093/bja/aei009. [DOI] [PubMed] [Google Scholar]
- 7.Karakaya D, Buyukgoz F, Baris S, Guldogus F, Tur A. Addition of fentanyl to bupivacaine prolongs anesthesia and analgesia in axillary brachial plexus block. Reg Anesth Pain Med. 2001;26:434–8. doi: 10.1053/rapm.2001.24675. [DOI] [PubMed] [Google Scholar]
- 8.Fanelli G, Casati A, Magistris L, Berti M, Albertin A, Scarioni M, et al. Fentanyl does not improve the nerve block characteristics of axillary brachial plexus anesthesia performed with ropivacaine. Acta Anaesthesiol Scand. 2001;45:590–4. doi: 10.1034/j.1399-6576.2001.045005590.x. [DOI] [PubMed] [Google Scholar]
- 9.Clerc S, Vuillermier H, Frascarolo P, Spahn DR, Gardaz J. Is the effect of inguinal field block with 0.5% bupivacaine on postoperative pain after hernia repair enhanced by addition of ketorolac or S(+) ketamine? Clin J Pain. 2005;21:101–5. doi: 10.1097/00002508-200501000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Noyan A. On effects of ketamine to axillary block in hand surgery. J Reconstr Microsurg. 2002;18:197. doi: 10.1055/s-2002-28472. [DOI] [PubMed] [Google Scholar]
- 11.Jarbo K, Batra YK, Panda NB. Brachial plexus block with midazolam and bupivacaine improves analgesia. Can J Anaesth. 2005;52:822–6. doi: 10.1007/BF03021776. [DOI] [PubMed] [Google Scholar]
- 12.Murphy DB, McCartney CJ, Chan VW. Novel analgesic adjuvants for brachial plexus block, a systemic review. Anesth Analg. 2000;90:1122–8. doi: 10.1097/00000539-200005000-00023. [DOI] [PubMed] [Google Scholar]
- 13.Neal JM, Gerancher JC, Hebel JR, Ilfeld BM, McCartney CJ, Franco CD, et al. Upper extremity regional anesthesia-essentials of our current understanding. Reg Anesth Pain Med. 2009;34:134–70. doi: 10.1097/AAP.0b013e31819624eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colombo G, Padera R, Langer R, Kohane DS. Prolonged duration local anesthesia with lipid-protein-sugar particles containing bupivacaine and dexamethasone. J Biomed Mater Res A. 2005;75:458–64. doi: 10.1002/jbm.a.30443. [DOI] [PubMed] [Google Scholar]
- 15.Drager C, Benziger D, Gao F, Berde CB. Prolonged intercostal nerve blockade in sheep using controlled-release of bupivacaine and dexamethasone from polymer microspheres. Anesthesiology. 1998;89:969–79. doi: 10.1097/00000542-199810000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Movafegh A, Razazian M, Hajimaohamadi F, Meysamie A. Dexamethasone added to lidocaine prolongs axillary brachial plexus blockade. Anesth Analg. 2006;102:263–7. doi: 10.1213/01.ane.0000189055.06729.0a. [DOI] [PubMed] [Google Scholar]
- 17.Parrington SJ, O’Donnell D, Chan VW, Brown-Shreves D, Subramanyam R, Qu M, et al. Dexamethasone added to mepivacaine prolongs the duration of analgesia after supraclavicular brachial plexus blockade. Reg Anesth Pain Med. 2010;35:422–6. doi: 10.1097/AAP.0b013e3181e85eb9. [DOI] [PubMed] [Google Scholar]
- 18.Shrestha BR, Maharjan SK, Tabedar S. Supraclavicular brachial plexus block with and without dexamethasone - A comparative study. Kathmandu Univ Med J. 2003;1:158–60. [PubMed] [Google Scholar]
- 19.Vieira PA, Pulai I, Tsao GC, Manikantan P, Keller B, Connelly NR. Dexamethasone with bupivacaine increases duration of analgesia in ultrasound-guided interscalene brachial plexus blockade. Eur J Anaesthesiol. 2010;27:285–8. doi: 10.1097/EJA.0b013e3283350c38. [DOI] [PubMed] [Google Scholar]
- 20.Cummings KC, 3rd, Napierkowski DE, Parra-Sanchez I, Kurz A, Dalton JE, Brems JJ, et al. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. Br J Anaesth. 2011;107:446–53. doi: 10.1093/bja/aer159. [DOI] [PubMed] [Google Scholar]
- 21.Pathak RG, Anand PS, Rajendra NK. Supraclavicular brachial plexus block with and without Dexamethasone – A Comparative Study. Int J Sci Res Publ. 2012;12:1–7. [Google Scholar]
- 22.Yasuda I, Hirano T, Ojima T. Supraclavicular brachial plexus block using a nerve stimulator and an insulated needle. Br J Anaesth. 1980;52:409–11. doi: 10.1093/bja/52.4.409. [DOI] [PubMed] [Google Scholar]
- 23.Golwala M, Swadia V, Dhimar A, Sridahr N. Pain relief by dexamethasone as an adjuvant to local anaesthetics in supraclavicular brachial plexus block. J Anaesth Clin Pharmacol. 2009;25:285–8. [Google Scholar]
- 24.Castillo J, Curley J, Hotz J, Uezono M, Tigner J, Chasin M, et al. Glucocorticoids prolong rat sciatic nerve blockade in vivo from bupivacaine microspheres. Anesthesiology. 1996;85:1157–66. doi: 10.1097/00000542-199611000-00025. [DOI] [PubMed] [Google Scholar]
- 25.Kopacz DJ, Lacouture PG, Wu D, Nandy P, Swanton R, Landau C. The dose response and effects of dexamethasone on bupivacaine microcapsules for intercostal blockade (T9 to T11) in healthy volunteers. Anesth Analg. 2003;96:576–82. doi: 10.1097/00000539-200302000-00050. [DOI] [PubMed] [Google Scholar]
- 26.McCormack K. The spinal actions of nonsteroidal anti-inflammatory drugs and the dissociation between their anti-inflammatory and analgesic effects. Drugs. 1994;47:28–45. doi: 10.2165/00003495-199400475-00006. [DOI] [PubMed] [Google Scholar]
- 27.Ahlgren SC, Wang JF, Levine JD. C-fiber mechanical stimulus response functions are different in inflammatory versus neuropathic hyperalgesia in the rat. Neuroscience. 1997;76:285–90. doi: 10.1016/s0306-4522(96)00290-4. [DOI] [PubMed] [Google Scholar]
- 28.Honorio TB. Epidural Steroids: In P. Prithvi Raj. Pain medicine, a comprehensive review. Mosby publications. 1999:259–63. [Google Scholar]
- 29.Attardi B, Takimoto K, Gealy R. Glucocorticoid induced upregulation of a pituitary K+ channel mRNA in vitro and in vivo. Receptors Channels. 1993;1:287–93. [PubMed] [Google Scholar]
- 30.Tan PH, Liu K, Peng CH, Yang LC, Lin CR, Lu CY. The effect of dexamethasone on postoperative pain and emesis after intrathecal neostigmine. Anesth Analg. 2001;92:228–32. doi: 10.1097/00000539-200101000-00044. [DOI] [PubMed] [Google Scholar]
- 31.Splinter WM, Rhine EJ. Low-dose ondansetrone with dexamethasone more effectively decreases vomiting after strabismus surgery in children than does high-dose ondansetron. Anesthesiology. 1998;88:72–5. doi: 10.1097/00000542-199801000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Neal JM, Rathmell JP, Rowlingson JC. Publishing studies that involve ‘Off-label’ use of drugs: Formalizing regional anesthesia and pain medicine's policy. Reg Anesth Pain Med. 2009;34:391–2. doi: 10.1097/AAP.0b013e3181b87066. [DOI] [PubMed] [Google Scholar]
- 33.Benzon HT, Gissen AJ, Strichartz GR, Avram MJ, Covino BG. The effect of polyethylene glycol on mammalian nerve impulses. Anesth Analg. 1987;66:553–9. [PubMed] [Google Scholar]
- 34.Benzon HT, Chew TL, McCarthy RJ, Benzon HA, Walega DR. Comparison of the particle sizes of different steroids and the effect of dilution: A review of the relative neurotoxicities of the steroids. Anesthesiology. 2007;106:331–8. doi: 10.1097/00000542-200702000-00022. [DOI] [PubMed] [Google Scholar]
- 35.Aasboe V, Raeder JC, Groegaard B. Betamethasone reduces postoperative pain and nausea after ambulatory surgery. Anesth Analg. 1998;87:913–7. doi: 10.1097/00000539-199808000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Baxendale BR, Vater M, Lavery KM. Dexamethasone reduces pain and swelling following extraction of third molar teeth. Anaesthesia. 1993;48:961–4. doi: 10.1111/j.1365-2044.1993.tb07474.x. [DOI] [PubMed] [Google Scholar]
- 37.Liu K, Hsu CC, Chia YY. Effect of dexamethasone on postoperative pain and emesis. Br J Anaesth. 1998;80:85–6. doi: 10.1093/bja/80.1.85. [DOI] [PubMed] [Google Scholar]
- 38.Pasternak JJ, McGregor DG, Lanier WL. Effect of single-dose dexamethasone on blood glucose concentration in patients undergoing craniotomy. J Neurosurg Anesthesiol. 2004;16:122–5. doi: 10.1097/00008506-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Moore D. Supraclavicular approach for block of the brachial plexus. In: Moore D, editor. Regional block. A handbook for use in the clinical practice of medicine and surgery. 4th ed. Springfield: Charles C Thomas Publisher; 1981. pp. 221–42. [Google Scholar]
- 40.Lanz E, Theiss D, Jankovic D. The extent of blockade following various techniques of brachial plexus block. Anesth Analg. 1983;62:55–8. [PubMed] [Google Scholar]
- 41.Urmey W. Upper extremity blocks. In: Brown D, editor. Regional anesthesia and analgesia. Philadelphia: WB Saunders Company; 1996. pp. 254–78. [Google Scholar]


