Abstract
Newborns have an innate system for preferentially looking at an upright human face. This face preference behaviour disappears at approximately one month of age and reappears a few months later. However, the neural mechanisms underlying this U-shaped behavioural change remain unclear. Here, we isolate the functional development of the cortical visual pathway for face processing using S-cone-isolating stimulation, which blinds the subcortical visual pathway. Using luminance stimuli, which are conveyed by both the subcortical and cortical visual pathways, the preference for upright faces was not observed in two-month-old infants, but it was observed in four- and six-month-old infants, confirming the recovery phase of the U-shaped development. By contrast, using S-cone stimuli, two-month-old infants already showed a preference for upright faces, as did four- and six-month-old infants, demonstrating that the cortical visual pathway for face processing is already functioning at the bottom of the U-shape at two months of age. The present results suggest that the transient functional deterioration stems from a conflict between the subcortical and cortical functional pathways, and that the recovery thereafter involves establishing a level of coordination between the two pathways.
Keywords: S cone, face perception, subcortical network
1. Introduction
Neonates with an immature visual cortex prefer to look at an upright human face and a schematic face immediately after a birth [1–4]. Although the neural basis of this preference remains uncertain, Johnson proposed that a subcortical visual pathway, including the superior colliculus and pulvinar, is involved in this innate preference for face configuration [1,5]. This face preference disappears around six weeks after birth, and it reappears at a few months of age, accompanied by more sophisticated face-processing abilities [1,2,6]. Cortical responses to the face inversion effect are also observed beginning at three months of age [7]. Therefore, it has generally been hypothesized that this U-shaped behavioural change reflects a hierarchical shift from the innate subcortical network to the acquired cortical network during early infancy [2]. According to this theory, the subcortical functions wane or are inhibited by the cerebral cortex after one month of age. However, this theory contradicts several lines of evidence supporting that the subcortical visual pathway still contributes to face processing in human adults [8–10]. This issue raises the possibility that the face-specialized neural network develops through interactions between the subcortical and cortical visual pathways. Here, we propose that the transient conflict between the two functional pathways produces the bottom of the U-shaped change in face-preference behaviour, and that the recovery thereafter reflects the establishment of coordination between the two pathways.
Visual information defined by luminance is conveyed to both the subcortical and cortical visual pathways. Therefore, it is difficult to distinguish which brain networks play a proactive role in infants' face-preference behaviour. In contrast to luminance information, stimuli activating only the short-wave sensitivity cone (S cone) in the retina are exclusively conveyed through the geniculocortical pathway [11–14]. By applying this stimulation method to facial information, we previously showed that rapid facial detection in human adults, compared with the detection of other objects, was achieved by the subcortical visual pathway, but not by the cortical pathway [10]. Therefore, by using this method in this study, we investigated the functional development of the subcortical and cortical networks and their interaction during face processing in early infancy.
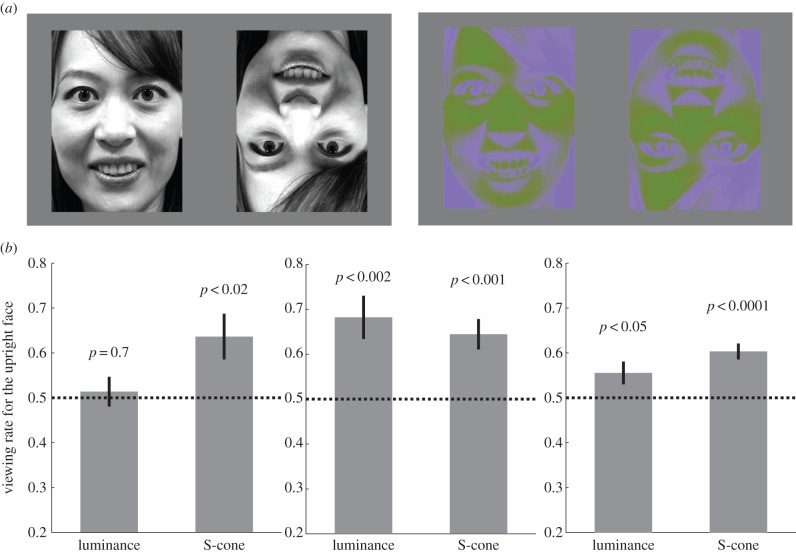
Although the S-cone visual pathway is immature at birth, two-month olds develop cone sensitivity that is comparable to that of adults [15]. Brain responses to S-cone stimuli also emerge at two months of age [16,17]. These facts suggest that younger infants may not respond properly to the S-cone stimuli per se. Therefore, this study examined infants' preferences for an upright female face compared with an inverted face using luminance and S-cone stimuli in two-, four- and six-month-old infants (figure 1a). A preference for the S-cone-defined upright face indicated that the face-specialized cortical network was already functional, with no aid from the subcortical network, at this age.
Figure 1.
Preference rate for the upright face in young infants. (a) The face images taken from the video clip used in the study: the female face represented in the luminance (left panel) and the S-cone stimuli (right panel). (b) The viewing rate for the upright face (versus the inverted face) with the luminance and the S-cone stimuli of two-, four- and six-month-old infants. The dotted line represents chance (0.5).
2. Material and methods
(a). Participants
The participants consisted of 50 healthy, full-term infants divided into three groups based on age: 16 two-month olds (mean age: 79 days, range: 68–88 days; nine males), 17 four-month olds (mean age: 135 days, range: 122–146 days; eight males) and 17 six-month olds (mean age: 202 days, range: 193–213 days; six males). Informed consent was obtained from the parents. An additional 14 infants were excluded from the final sample because of gaze position bias (more than 85% of the viewing time; 9 two-month olds, 1 six-month old) and fussiness (1 two-month old, 2 four-month olds, 1 six-month old).
(b). Apparatus
The infants sat on an adult's lap at a distance of 50 cm from a gamma-corrected liquid crystal 23-inch monitor (ColorEdge CS230, Eizo, Japan). The spatial resolution of the monitor screen was 1920 × 1080 pixels (45° × 25°). The infant's eye level was aligned to the centre of the screen at the appropriate height. The person holding the baby fixated at the centre of the upper side of the monitor throughout the session. A video camera was mounted just below the monitor and was masked from the infant. Magnified videos of the infant's face were recorded on the hard disk of the video recorder (BDZ-ED2000, Sony, Japan). The infant's eye movements were later analysed frame by frame by two scorers who were blinded to the presented stimulus and who were unaware of the aim of this study. They assessed the infant's gaze direction according to four choices: left side, centre, right side or look away. The mean agreement rate between the two scorers was 88%, and the data with incongruent judgements were excluded from further analyses.
(c). Stimuli
Infants successively viewed four video clips with sound, which each lasted for 18 s. Each video clip consisted of the upright and inverted faces of a woman singing a Japanese nursery rhyme, with one face on the left and the other face on the right side of the screen (written informed consent to publish the photograph has been obtained from the model). The original full-colour video clip was first converted to greyscale (0–255, mean = 127) and resized to 10.5° × 14.6°. The greyscale video images were used as the luminance-defined stimuli (figure 1a). To prepare the S-cone-isolating stimuli, the greyscale video clip was linearly converted on the S-cone-isolating line over the range of S-cone excitation levels that the monitor was capable of producing (figure 1a). Applying the same procedures as our previous study [10], the S-cone-isolating line was calculated using the spectral absorptions of the human L, M and S cones [18], and the spectral emissions of the red, green and blue phosphors of the monitor were measured using a spectral calorimeter (CS-2000, Konica Minolta, Japan). We used the spectral absorptions of adult humans, because previous studies have demonstrated that the S-cone sensitivity of two-month-old infants has already developed to the same level as that of adults [15–17].
The face videos were presented on their respective sides at a distance of 5.9° from the centre for four-month olds and six-month olds and at a distance of 3.5° from the centre for two-month olds. The distance between the two pictures was narrowed for two-month olds because they did not show gaze shifts between the two videos at a distance of 5.9° in preliminary tests. In each infant group, half of the infants viewed the video clips in the following order: luminance stimuli with the upright face on the right, S-cone stimuli with the upright face on the left, luminance stimuli with the upright face on the left and S-cone stimuli with the upright face on the right; the other half of the infants were shown the videos in the order of: S-cone stimuli with the upright face on the left, luminance stimuli with the upright face on the right, S-cone stimuli with the upright face on the right and luminance stimuli with the upright face on the left. Checkerboard reversal stimuli with mechanical sounds were presented at the centre of the screen for 1.8 s before the presentation of each video clip to attract the infant's fixation to the centre.
3. Results
In all of the infant groups, the infants viewed the screen for a duration of more than 70% of the total movie lengths (36 s) on average, irrespective of whether the luminance-defined (two-month olds: 78 ± 4% [mean ± 1 s.e.]; four-month olds: 90 ± 2%; six-month olds: 83 ± 3%) or S-cone-isolating stimuli were presented (two-month olds: 74 ± 3%; four-month olds: 84 ± 3%; six-month olds: 72 ± 4%). In addition, the two-month olds switched their gaze positions between the left and the right face stimuli with the luminance (the number of switches 22.3 ± 3.7 [mean ± 1 s.e.]) and the S-cone stimuli (17.6 ± 2.6) as well as four-month olds (luminance: 9.2 ± 1.3; S-cone: 21.1 ± 2.3) and six-month olds (luminance: 17.6 ± 1.2; S-cone: 28.5 ± 1.5) while viewing the videos.
As shown in figure 1b, the viewing rate for the luminance-defined upright face was not different from the chance level (0.5) in the two-month olds (0.51 ± 0.03; t15 = 0.4, p = 0.7, two-tailed Student's t-test), but it was significantly greater than the chance level in the four-month olds (0.68 ± 0.05, t16 = 3.8, p < 0.002) and in the six-month olds (0.56 ± 0.03, t16 = 2.2, p < 0.05). By contrast, the viewing rate for the S-cone-defined upright face was significantly greater than the chance level in all of the age groups: 0.64 ± 0.05 for the two-month olds (t15 = 2.7, p < 0.02), 0.64 ± 0.03 for the four-month olds (t16 = 4.3, p < 0.001) and 0.60 ± 0.02 for the six-month olds (t16 = 5.9, p < 0.0001).
4. Discussion
With the luminance-defined stimuli, two-month-old infants did not show any preference for the upright face, but the preference was recovered in four- and six-month olds. According to previous studies, neonates are born with a preference for upright faces [1–3], but this responsiveness to facial configuration is transiently lost at approximately one to two months of age [1,6]. Collectively, the results of both our study and previous studies indicate that the preference for upright faces changes in a U-shaped manner, with the bottom occurring at one to two months of age, as long as luminance-defined stimuli are used.
By contrast, when the S-cone-isolating stimuli were presented, the two-month-old infants already showed a preference for the upright face, despite being at the bottom of the U-shaped curve for luminance-defined stimuli. The difference between the luminance and S-cone stimuli is that the S-cone stimuli are not conveyed by the retinotectal subcortical pathway [11]. One might argue that the subcortical visual pathway is involved in colour processing, because the pulvinar in monkeys receives direct projections from all types of colour-opponent ganglion cells [19,20]. However, previous behavioural and fMRI studies on patients who underwent hemispherectomies clearly refute this possibility, because blindsight is mediated by the S-cone-independent subcortical pathway [21,22]. These findings indicate that the S-cone stimuli are exclusively conveyed by the cortical visual pathway in humans. Thus, the preference for upright faces using the S-cone stimuli demonstrates that the cortical network is already functioning to process facial orientation with no aid from the subcortical system two months after birth.
This raises the question of why the two-month-old infants failed to show any preference for face orientation when presented with the luminance-defined stimuli, which activate both the subcortical and cortical visual pathways. As discussed, the innate subcortical network is able to discriminate face orientation at birth [1–3]. Many previous studies have shown that the subcortical pathway for face orientation survives into adulthood [10,23–26]. Thus, the subcortical pathway should maintain its ability to discriminate face orientation even at two months of age. The transient loss of this preference for upright faces at two months of age may result from a conflict between the subcortical and cortical pathways, each of which would be functional if the other system were blinded. Accordingly, the subsequent recovery of the preference for upright faces by four and six months of age can be interpreted as an establishment of coordination between the two systems.
U-shaped changes in a given behaviour, i.e. where there is a transient loss and subsequent recovery of the behaviour, are observed in many behaviours such as reaching [27], sound orientation [28,29] and cross modal integration [30]. These U-shaped changes in behaviour are generally believed to reflect a shift in the neural basis from the innate subcortical network to the postnatally acquired cortical network [5]. Against this hierarchical developmental view, we suggest that the lack of coordination between the subcortical and cortical networks produces a transient functional deterioration, and the recovery thereafter reflects the process of establishing coordination between the two pathways, both of which are then functional into adulthood (figure 2).
Figure 2.
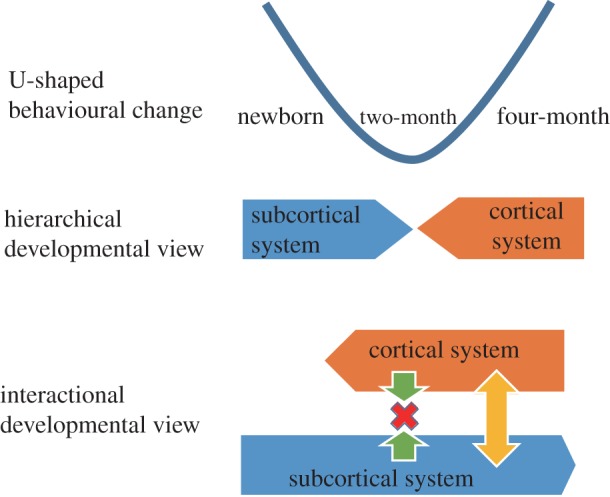
Schematics of brain developmental views underlying the U-shaped behavioural change during early infancy.
One might argue that the upright face preference demonstrated with the S-cone stimuli resulted from a domain-general perceptual bias rather than from a face-specific bias. Indeed, previous studies clearly show that the upright face preference in neonates is driven by a domain-general perceptual bias towards top-heavy patterns [3,31]. However, this general mechanism could not explain the upright face preference at three months of age, because the infants preferred the upright normal face, but not the top-heavy unnatural face [32]. Consistently, face-specific cortical responses emerge in two- to three-month olds [33,34]. These facts suggest that the cortical network is already specialized for processing the human face by this age. Because the S-cone stimuli are exclusively conveyed by the cortical visual pathway, the upright face preference demonstrated for the S-cone stimuli is likely driven by the face-specific perceptual bias.
One limitation of this study is that our paradigm may not be optimal for eliciting collicular responses, which primarily mediate the act of orienting to peripheral stimuli, resulting in the disappearance of the upright face preference when the two-month-old infants were presented with the luminance-defined stimuli. This study showed faces in foveal positions for two reasons. First, S cones are distributed in the foveal areas of the retina. Second, we examined the preference behaviour by comparing the looking duration, but not the number of orienting responses across all age groups. When the pairs of stimuli are separated by a wide distance, the young infants could not shift their gaze between them. However, it might be necessary to examine whether the disappearance of the upright face preference with the luminance-defined stimuli depends on the stimulus position.
The present results suggest that the brain network for face processing is constructed through interactions between the subcortical and cortical networks during postnatal life. Individuals with autism spectrum disorder (ASD), which is characterized by impairments in social cognition and communication, show atypical gaze patterns towards human faces [35,36]. Especially, the lack of rapid and automatic social orientation in ASD raised the hypothesis that a deficit in the subcortical neural pathway causes the eventual widespread social dysfunction [37–39]. However, several recent studies refute this hypothesis and demonstrate that the innate subcortical route for social orienting is intact in ASD [40]; Shah et al. [41] reported that adults with ASD exhibit robust automatic orienting to protofacial stimuli, which involves the subcortical visual pathway. Furthermore, infants who were later diagnosed with ASD showed a high preference for viewing the eyes at two months of age. However, these infants exhibited a decline in eye-looking behaviour from two to six months of age, whereas those with typical development demonstrated an increase in eye-viewing behaviour [42]. Assuming that this period is critical for reorganizing the subcortical and cortical networks for face perception, it is possible that the decline in eye-looking behaviour in ASD resulted from a lack of coordination between the subcortical and cortical systems. Our method of using S-cone-isolating stimuli may be useful for investigating this issue in the future.
Supplementary Material
Acknowledgements
We are grateful to Shigeru Kitazawa, Uta Frith and Gergely Csibra for their detailed comments on a draft of this article.
The study was approved by the ethical committee of Osaka University.
Data accessibility
The datasets supporting this article have been uploaded as the electronic supplementary material.
Funding statement
This work was supported by the grant in aid for Scientific Research on Innovative Areas 251195040 ‘Constructive Developmental Science’ from the Ministry of Education, Culture, Sports, Science and Technology, Japan to T. N.
References
- 1.Johnson MH, Dziurawiec S, Ellis H, Morton J. 1991. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition 40, 1–19. ( 10.1016/0010-0277(91)90045-6) [DOI] [PubMed] [Google Scholar]
- 2.Mondloch CJ, Lewis TL, Budreau DR, Maurer D, Dannemiller JL, Stephens BR, Kleiner-Gathercoal KA. 1999. Face perception during early infancy. Psychol. Sci. 10, 419–422. ( 10.1111/1467-9280.00179) [DOI] [Google Scholar]
- 3.Cassia VM, Turati C, Simion F. 2004. Can a nonspecific bias toward top-heavy patterns explain newborns’ face preference? Psychol. Sci. 15, 379–383. ( 10.1111/j.0956-7976.2004.00688.x) [DOI] [PubMed] [Google Scholar]
- 4.Di Giorgio E, Leo I, Pascalis O, Simion F. 2012. Is the face-perception system human-specific at birth? Dev. Psychol. 48, 1083–1090. ( 10.1037/a0026521) [DOI] [PubMed] [Google Scholar]
- 5.Johnson MH. 2005. Subcortical face processing. Nat. Rev. Neurosci. 6, 766–774. ( 10.1038/nrn1766) [DOI] [PubMed] [Google Scholar]
- 6.Maurer D, Barrera M. 1981. Infants’ perception of natural and distorted arrangements of a schematic face. Child Dev. 52, 196–202. ( 10.2307/1129230) [DOI] [PubMed] [Google Scholar]
- 7.Hoehl S, Peykarjou S. 2012. The early development of face processing: what makes faces special? Neurosci. Bull. 28, 765–788. ( 10.1007/s12264-012-1280-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vuilleumier P, Armony JL, Driver J, Dolan RJ. 2003. Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nat. Neurosci. 6, 624–631. ( 10.1038/nn1057) [DOI] [PubMed] [Google Scholar]
- 9.Morris JS, DeGelder B, Weiskrantz L, Dolan RJ. 2001. Differential extrageniculostriate and amygdala responses to presentation of emotional faces in a cortically blind field. Brain 124, 1241–1252. ( 10.1093/brain/124.6.1241) [DOI] [PubMed] [Google Scholar]
- 10.Nakano T, Higashida N, Kitazawa S. 2013. Facilitation of face recognition through the retino-tectal pathway. Neuropsychologia 51, 2043–2049. ( 10.1016/j.neuropsychologia.2013.06.018) [DOI] [PubMed] [Google Scholar]
- 11.Sumner P, Adamjee T, Mollon JD. 2002. Signals invisible to the collicular and magnocellular pathways can capture visual attention. Curr. Biol. 12, 1312–1316. ( 10.1016/S0960-9822(02)01020-5) [DOI] [PubMed] [Google Scholar]
- 12.Marrocco RT, Li RH. 1977. Monkey superior colliculus: properties of single cells and their afferent inputs. J. Neurophysiol. 40, 844–860. [DOI] [PubMed] [Google Scholar]
- 13.Schiller PH, Malpeli JG. 1977. Properties and tectal projections of monkey retinal ganglion cells. J. Neurophysiol. 40, 428–445. [DOI] [PubMed] [Google Scholar]
- 14.White BJ, Boehnke SE, Marino RA, Itti L, Munoz DP. 2009. Color-related signals in the primate superior colliculus. J. Neurosci. 29, 12 159–12 166. ( 10.1523/JNEUROSCI.1986-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobson V. 1976. Spectral sensitivity of the 2-month infant as measured by the visually evoked cortical potential. Vision Res. 16, 367–374. ( 10.1016/0042-6989(76)90198-X) [DOI] [PubMed] [Google Scholar]
- 16.Suttle CM, Banks MS, Graf EW. 2002. FPL and sweep VEP to tritan stimuli in young human infants. Vision Res. 42, 2879–2891. ( 10.1016/S0042-6989(02)00333-4) [DOI] [PubMed] [Google Scholar]
- 17.Suttle CM, Anderson SJ, Harding GF. 1997. A longitudinal study of visual evoked responses to tritan stimuli in human infants. Optom. Vis. Sci. 74, 717–725. ( 10.1097/00006324-199709000-00019) [DOI] [PubMed] [Google Scholar]
- 18.Smith VC, Pokorny J. 1975. Spectral sensitivity of the foveal cone photopigments between 400 and 500 nm. Vision Res. 15, 161–171. ( 10.1016/0042-6989(75)90203-5) [DOI] [PubMed] [Google Scholar]
- 19.Felsten G, Benevento LA, Burman D. 1983. Opponent-color responses in macaque extrageniculate visual pathways: the lateral pulvinar. Brain Res. 288, 363–367. ( 10.1016/0006-8993(83)90119-1) [DOI] [PubMed] [Google Scholar]
- 20.Cowey A, Stoerig P, Bannister M. 1994. Retinal ganglion cells labelled from the pulvinar nucleus in macaque monkeys. Neuroscience 61, 691–705. ( 10.1016/0306-4522(94)90445-6) [DOI] [PubMed] [Google Scholar]
- 21.Leh SE, Ptito A, Schonwiesner M, Chakravarty MM, Mullen KT. 2010. Blindsight mediated by an S-cone-independent collicular pathway: an fMRI study in hemispherectomized subjects. J. Cogn. Neurosci. 22, 670–682. ( 10.1162/jocn.2009.21217) [DOI] [PubMed] [Google Scholar]
- 22.Leh SE, Mullen KT, Ptito A. 2006. Absence of S-cone input in human blindsight following hemispherectomy. Eur. J. Neurosci. 24, 2954–2960. ( 10.1111/j.1460-9568.2006.05178.x) [DOI] [PubMed] [Google Scholar]
- 23.Tamietto M, de Gelder B. 2010. Neural bases of the non-conscious perception of emotional signals. Nat. Rev. Neurosci. 11, 697–709. ( 10.1038/nrn2889) [DOI] [PubMed] [Google Scholar]
- 24.Tomalski P, Johnson MH, Csibra G. 2009. Temporal-nasal asymmetry of rapid orienting to face-like stimuli. Neuroreport 20, 1309–1312. ( 10.1097/WNR.0b013e32832f0acd) [DOI] [PubMed] [Google Scholar]
- 25.Tomalski P, Csibra G, Johnson MH. 2009. Rapid orienting toward face-like stimuli with gaze-relevant contrast information. Perception 38, 569–578. ( 10.1068/p6137) [DOI] [PubMed] [Google Scholar]
- 26.Stein T, Sterzer P. 2012. Not just another face in the crowd: detecting emotional schematic faces during continuous flash suppression. Emotion 12, 988–996. ( 10.1037/a0026944) [DOI] [PubMed] [Google Scholar]
- 27.Butterworth GE. 1989. On U-shaped and other transitions in sensori-motor development. In Transition mechanisms in child development (ed. de Ribaupierre A.), pp. 283–296. New York: Cambridge University Press. [Google Scholar]
- 28.Muir DW, Clifton RK, Clarkson MG. 1989. The development of a human auditory localization response: a U-shaped function. Can. J. Psychol. 43, 199–216. ( 10.1037/h0084220) [DOI] [PubMed] [Google Scholar]
- 29.Field J, Muir D, Pilon R, Sinclair M, Dodwell P. 1980. Infants’ orientation to lateral sounds from birth to three months. Child Dev. 51, 295–298. ( 10.2307/1129628) [DOI] [PubMed] [Google Scholar]
- 30.Taga G, Ikejiri T, Tachibana T, Shimojo S, Soeda A, Takeuchi K, Konishi Y. 2002. Visual feature binding in early infancy. Perception 31, 273–286. ( 10.1068/p3167) [DOI] [PubMed] [Google Scholar]
- 31.Turati C, Simion F, Milani I, Umilta C. 2002. Newborns’ preference for faces: what is crucial? Dev. Psychol. 38, 875–882. ( 10.1037/0012-1649.38.6.875) [DOI] [PubMed] [Google Scholar]
- 32.Turati C, Valenza E, Leo I, Simion F. 2005. Three-month-olds’ visual preference for faces and its underlying visual processing mechanisms. J. Exp. Child Psychol. 90, 255–273. ( 10.1016/j.jecp.2004.11.001) [DOI] [PubMed] [Google Scholar]
- 33.Tzourio-Mazoyer N, De Schonen S, Crivello F, Reutter B, Aujard Y, Mazoyer B. 2002. Neural correlates of woman face processing by 2-month-old infants. Neuroimage 15, 454–461. ( 10.1006/nimg.2001.0979) [DOI] [PubMed] [Google Scholar]
- 34.Halit H, de Haan M, Johnson MH. 2003. Cortical specialisation for face processing: face-sensitive event-related potential components in 3- and 12-month-old infants. Neuroimage 19, 1180–1193. ( 10.1016/S1053-8119(03)00076-4) [DOI] [PubMed] [Google Scholar]
- 35.Nakano T, Tanaka K, Endo Y, Yamane Y, Yamamoto T, Nakano Y, Ohta H, Kato N, Kitazawa S. 2010. Atypical gaze patterns in children and adults with autism spectrum disorders dissociated from developmental changes in gaze behaviour. Proc. R. Soc. B 277, 2935–2943. ( 10.1098/rspb.2010.0587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klin A, Jones W, Schultz R, Volkmar F, Cohen D. 2002. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch. Gen. Psychiatry 59, 809–816. ( 10.1001/archpsyc.59.9.809) [DOI] [PubMed] [Google Scholar]
- 37.Senju A, Tojo Y, Dairoku H, Hasegawa T. 2004. Reflexive orienting in response to eye gaze and an arrow in children with and without autism. J. Child Psychol. Psychiatry Allied Discipl. 45, 445–458. ( 10.1111/j.1469-7610.2004.00236.x) [DOI] [PubMed] [Google Scholar]
- 38.Kleinhans NM, Richards T, Johnson LC, Weaver KE, Greenson J, Dawson G, Aylward E. 2011. fMRI evidence of neural abnormalities in the subcortical face processing system in ASD. Neuroimage 54, 697–704. ( 10.1016/j.neuroimage.2010.07.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dawson G, Webb SJ, McPartland J. 2005. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Dev. Neuropsychol. 27, 403–424. ( 10.1207/s15326942dn2703_6) [DOI] [PubMed] [Google Scholar]
- 40.Johnson MH. 2014. Autism: demise of the innate social orienting hypothesis. Curr. Biol. 24, R30–R31. ( 10.1016/j.cub.2013.11.021) [DOI] [PubMed] [Google Scholar]
- 41.Shah P, Gaule A, Bird G, Cook R. 2013. Robust orienting to protofacial stimuli in autism. Curr. Biol. 23, R1087–R1088. ( 10.1016/j.cub.2013.10.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones W, Klin A. 2013. Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature 504, 427–431. ( 10.1038/nature12715) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as the electronic supplementary material.