Abstract
OBJECTIVES:
To describe the immunogenicity and safety of a two-dose series of a quadrivalent meningococcal (serogroups A, C, Y and W) polysaccharide diphtheria toxoid conjugate vaccine (MenACYW-D) administered to toddlers.
METHODS:
Children were randomly assigned (1:1) at study entry to receive MenACYW-D at 12 and 18 months of age (group 1; n=61) or meningococcal serogroup C conjugate vaccine (MCC) at 12 months of age (group 2; n=62). All received routine childhood immunizations. A, C, Y and W antibody titres were measured in group 1 before and one month after the 18-month MenACYW-D vaccination and were measured in group 2 at one and seven months post-MCC vaccination. Antibodies elicited by diphtheria and tetanus toxoids, and acellular pertussis vaccine adsorbed combined with inactivated poliomyelitis vaccine and Haemophilus influenzae b conjugate (DTaP-IPV-Hib) vaccine coadministered at the 18-month vaccination were measured one month later. Safety data were collected.
RESULTS:
At 19 months of age, ≥96% in group 1 achieved protective titres for the four meningococcal serogroups after dose 2; 67% in group 2 exhibited protective titres against serogroup C 28 days after MCC vaccination at 12 months of age, declining to 27% seven months later. DTaP-IPV-Hib elicited high antibody concentrations/titres in groups 1 and 2, consistent with historical values. The safety profiles after each dose generated no unexpected safety signals; no serious adverse events were related to vaccination.
DISCUSSION:
A two-dose series of MenACYW-D given concomitantly with a DTaP-IPV-Hib booster dose at 18 months of age demonstrated a good immunogenicity and safety profile. A two-dose series of MenACYW-D can be used as an alternative to one dose of MCC and provides protection against additional serogroups (NCT ID: NCT01359449).
Keywords: Canada, Conjugate, Meningococcal, Quadrivalent, Vaccine
Abstract
OBJECTIFS :
Décrire l’immunogénicité et l’innocuité d’une série de deux doses du vaccin polysaccharadique conjugué quadrivalent contre le méningocoque (des sérogroupes A, C, Y et W) et l’anatoxine diphtérique (Men-ACYW-D) administrée aux tout-petits.
MÉTHODOLOGIE :
En début d’étude, les enfants ont été répartis au hasard (1:1) entre l’administration du vaccin Men-ACYW-D à 12 et 18 mois (groupe 1; n=61) ou du vaccin conjugué contre le méningocoque de sérogroupe C (Men-C-C) à 12 mois (groupe 2; n=62). Tous ont reçu les vaccins systématiques pour les enfants. Les chercheurs ont mesuré les titres d’anticorps A, C, Y et W dans le groupe 1 avant et un mois après l’administration du vaccin Men-ACYW-D à 18 mois et dans le groupe 2 un et sept mois après l’administration du vaccin Men-C-C. Un mois plus tard, ils ont mesuré les anticorps induits par les anatoxines diphtérique et tétanique et par le vaccin adsorbé contre la coqueluche acellulaire combiné au vaccin inactivé contre la poliomyélite et au vaccin conjugué contre l’Haemophilus influenzae de type b (DCaT-VPI- Hib) coadministrés lors du vaccin de 18 mois. Ils ont colligé des données d’innocuité.
RÉSULTATS :
À 19 mois, au moins 96 % des enfants du groupe 1 avaient des titres protecteurs contre les quatre sérogroupes du méningocoque après la dose 2, tandis que 67 % de ceux du groupe 2 présentaient des titres protecteurs contre le sérogroupe C 28 jours après le vaccin Men-C-C à 12 mois, reculant à 27 % sept mois plus tard. Le vaccin DCaT-VPI-Hib conférait de fortes concentrations et titres d’anticorps dans les groupes 1 et 2, conformément aux valeurs antérieures. Les profils d’innocuité après chaque dose ne s’associaient à aucun signe d’innocuité inattendu, et aucun événement indésirable grave n’était lié à la vaccination.
EXPOSÉ :
Une série de deux doses du vaccin Men-ACYW-D administrée en même temps que la dose de rappel du DCaT-VPI-Hib à 18 mois présente un bon profil d’immunogénicité et d’innocuité. Elle peut remplacer une dose du vaccin Men-C-C et conférer une protection contre des sérogroupes supplémentaires (ID NCT : NCT01359449).
Meningococci colonize the nasopharynx of 10% to 20% of healthy adults. Although only a small proportion of carriers develop invasive meningococcal disease (IMD), Neisseria meningitidis is nevertheless responsible for substantial worldwide morbidity and mortality, causing both epidemic and endemic disease. Worldwide incidence varies widely from fewer than one to three cases per 100,000 population in developed nations, to 10 to 25 cases per 100,000 in developing countries (1). The most common clinical presentations are meningococcemia and purulent meningitis, with nearly all clinical disease caused by five meningococcal serogroups: A, B, C, Y and W (formerly ‘W-135’ and now ‘W’, as per Harrison et al [2]) (2,3). Even with appropriate treatment, an overall mortality rate of 7% to 19% has been reported for IMD (4), with approximately 10% to 20% of recovering patients sustaining permanent disability (3,5,6).
In Canada, meningococcal illness has been a notifiable disease since the 1920s (7), with the annual incidence since the 1950s ranging from approximately 0.5 to 2 cases per 100,000 population. Serogroup B accounts for approximately one-half of all current cases, mostly in children and young adults, with remaining cases divided primarily between serogroups C, Y and W. A multicomponent meningococcal B vaccine was licensed in Canada in late 2013 (8).
Provincial vaccination programs against serogroup C were instituted during 2002 to 2005 to deal with outbreak clusters that had arisen in Canada since 1989. Variability in vaccination schedules evolved, such that in 2012, nine of 13 provinces and territories administer a single dose of monovalent serogroup C meningococcal conjugate vaccine (MCC) vaccine at 12 months of age, one province and two territories give one dose of MCC at two and 12 months of age, and one province gives one dose at two, four and 12 months of age. MCC vaccines (serogroup C polysaccharide antigen conjugated to CRM197 protein or tetanus toxoid) are administered throughout Canada, and all provincial jurisdictions administer a booster dose at 10 to <18 years of age of either MCC (seven jurisdictions) or quadrivalent ACWY conjugate vaccine (six jurisdictions) (9). As a result of these programs, the incidence of serogroup C IMD has decreased from 0.6 per 100,000 in 2001 to <0.1 per 100,000 between 2009 and 2011 (10). Since 2007, the incidence of IMD caused by serogroup C has been less than that of serogroup Y.
Although endemic incidence is low in Canada, the potential for outbreaks by any of the four non-B disease-causing serogroups is a concern. Such outbreaks are facilitated by global tourism, in which travellers can introduce more infectious and/or invasive forms of IMD. For example, the annual Hajj pilgrimage has been associated with outbreaks of IMD caused by various serogroups. There was a serogroup A outbreak in the late 1980s controlled with mandatory vaccination. An international outbreak of the previously rare W serogroup in 2000 to 2001 was associated with a new and hypervirulent form of IMD that had a high attack rate and a case fatality rate of 37% (11).
Future outbreak prevention may need to focus on vaccination against all major preventable serogroups and not only serogroup C if such outbreaks in Canada are due to non-B, non-C serogroups. The current study assesses the immunogenicity and safety of a meningococcal (serogroups A, C, Y and W) polysaccharide diphtheria toxoid conjugate vaccine (MenACYW-D [Menactra, Sanofi Pasteur, France]) given as two doses at 12 and 18 months of age in conjunction with routine recommended childhood immunizations. The present study was a descriptive study, and no preplanned hypotheses or comparisons were tested. The study was conducted within the context of the normal Quebec vaccination for this age group. Therefore, other routinely administered vaccines in Quebec were given, including pneumococcal (PCV13); measles, mumps, rubella, and varicella (MMRV) at 12 months of age; and measles, mumps and rubella (MMR) at 18 months of age (12). In Canada, MenACYW-D vaccine was licensed in May 2006 for administration to children, adolescents and adults two to 55 years of age (9) and, since June 2012, for infants nine to 23 months of age in a two-dose schedule, administered at least three months apart.
METHODS
The present study was an open-label, descriptive trial conducted at a single centre in Montreal, Quebec (NCT ID: NCT01359449). The primary objective was to describe the immunogenicity of two doses of MenACYW-D and a single dose of meningococcal conjugate serogroup C vaccine (MCC [Menjugate, Novartis, USA]) when administered with routine childhood immunizations. A secondary objective of the study was to describe the immunogenicity and safety profile of diphtheria and tetanus toxoids, and acellular pertussis vaccine adsorbed combined with inactivated poliomyelitis vaccine and Haemophilus b conjugate vaccine (tetanus protein conjugate) (DTaP-IPV-Hib [Pediacel, Sanofi Pasteur, France]) coadministered with MenACYW-D. In addition, the safety profiles of MenACYW-D and MCC when given concomitantly with routine vaccinations are described.
The study protocol, recruitment procedures and written information provided to participants were approved by the Montreal Children’s Hospital – McGill University Health Centre independent ethics committee and were conducted in accordance with the Declaration of Helsinki, Good Clinical Practice and applicable International Conference on Harmonization guidelines. In accordance with good clinical practice and local regulations, investigators’ institutional approval was obtained before the start of the trial. Written informed consent was obtained from the parents/guardians of all study participants before any procedures were performed. The manuscript was prepared following guidelines established by the Uniform Requirements for Manuscripts Submitted to Biomedical Journals.
Healthy, full-term children were eligible to participate if they were ≥12 months (365 to 400 days) of age, had not previously received a meningococcal vaccine and had no illness, risk factor or administrative complication (eg, scheduled participation in another trial) that could negatively impact trial validity or child health; full inclusion/exclusion criteria are described at ClinicalTrials.gov (13). Children were randomly assigned via a randomization list and permuted blocks to receive either MenACYW-D (group 1) or MCC (group 2).
Participants in group 1 received MenACYW-D at approximately 12 months (365 to 400 days) and 18 months (519 to 579 days) of age. Meningococcal vaccines were given along with routinely recommended vaccines for age, including an 18-month fourth-dose booster dose of DTaP-IPV-Hib. Group 2 participants received one dose of MCC at 12 months (365 to 400 days) of age administered with routinely recommended vaccines at 12 months of age. Other routinely administered vaccines in Quebec included PCV13 and MMRV at 12 months of age, and MMR at 18 months of age (12).
Blood collection and laboratory analysis
In group 1, blood samples were collected before dose 2 (18 month) MenACYW-D immunization given together with DTaP-IPV-Hib and at approximately 28 days after immunizations. According to the study protocol, the pre-dose 2 sample (18 month) was to be assayed only for a response to DTaP-IPV-Hib; however, through an erroneous scheduling of these pre-dose 2 samples for serum bactericidal assay assessment, the meningococcal response results were also collected. Because the status of the pre-dose 2 vaccination meningococcal response informs the results and conclusions, these data were included in the present study. Postvaccination blood samples were assessed for bactericidal activity against meningococcal serogroups A, C, W and Y. Pre- and postvaccination blood samples were also assessed for responses against diphtheria, tetanus, three polio types, five pertussis antigens (pertussis toxoid, pertactin, filamentous hemagglutinin and fimbriae 2/3) and Hib polyribosylribitol phosphate capsular polysaccharide.
In group 2, postvaccination samples were drawn one month and seven months after MCC immunization. Both samples were assessed for meningococcal bactericidal activity, and the seven-month sample was also assessed for antibodies elicited by DTaP-IPV-Hib.
All immunoassays were performed by Global Clinical Immunology at Sanofi Pasteur. Sample preparation and handling procedures were generally similar to those used by Pina et al (14). Meningococcal antibody titres were determined via serum bactericidal assay in the presence of baby rabbit complement (SBA-BR); titres ≥1:8 were considered to be protective. All assays were performed with established laboratory procedures for meningococcal vaccines described elsewhere (14) or DTaP-IPV-Hib (15).
Safety data
Study personnel recorded any reaction ≤30 min postvaccination. Using a dedicated, investigator-supplied diary card, parents/guardians recorded information about solicited reactions seven days after each meningococcal vaccination and information about unsolicited reactions 28 days postvaccination. Study personnel contacted parents/guardians by telephone eight days after immunization to ensure correct diary card completion; at 28 days postvaccination, study personnel reminded the parent/guardians of group 1 participants via telephone to return their completed diary card.
For tenderness, redness, swelling and fever, the intensity definitions (grade 1, grade 2 or grade 3) were similar to the study described by Pina et al (14). For vomiting, grade 1 is one episode/h, grade 2 is two to five episodes/h and grade 3 is ≥6 episodes/h. For abnormal crying, grade 1 is <1 h duration, grade 2 is 1 h to 3 h and grade 3 is ≥6 h. For drowsiness, grade 1 is sleepier than usual, grade 2 is did not wake up for a meal and grade 3 is sleeping most of the time (difficult to wake up). For irritability, grade 1 is easily consolable, grade 2 is requires increase attention and grade 3 is inconsolable.
Data analysis
Three datasets were defined for analysis. The full analysis set included all participants who received ≥1 dose of study vaccine and had not received any other vaccines (except influenza vaccine) ≤4 weeks before. The DTaP-IPV-Hib full analysis set included all full analysis set participants who also received three previous doses of DTaP-IPV-Hib. A safety analysis set was defined for each MenACYW-D or MCC dose and included all available safety data; therefore, the number of participants for dose 1 MenACYW-D, dose 2 MenACYW-D and the group 2 MCC dose varied.
A descriptive analysis of SBA-BR titres was conducted for each time point, vaccine group and serogroup. Meningococcal and DTaPIPV-Hib vaccines were assessed by the percentage of participants with titres equal to or greater than established levels of protection for each antigen and by geometric mean titres (GMT). The 95% CIs were calculated by either the normal approximation (GMTs) or the exact binomial distribution according to the Clopper-Pearson method (percentages). The assessment of antibody responses elicited by DTaP-IVP-Hib was analyzed essentially as described by Grimprel et al (15).
RESULTS
A total of 123 children (61 in group 1; 62 in group 2) were randomized at study entry. Ten participants in group 1 and three in group 2 did not meet entry criteria because their birth weight was <2500 g and/or they did not receive the three-dose infant DTaP-IPV-Hib primary vaccination series. Given that this occurrence was not believed to interfere with the immunogenicity results for the meningococcal vaccinations, these participants were retained in the full analysis set and safety set (but not in the DTaP-IPV-Hib full analysis set). Approximately 93% of enrollees completed the study, with four participants discontinuing the study in each group (Table 1). The age and sex distributions of the two groups were similar (Table 1). There were slightly more white children in group 1 (87% versus 74%), while black, Hispanic and Asian children represented ≤5% of each group. The second-largest ethnic demographic category was ‘other’, representing 8% of group 1 and 16% of group 2.
TABLE 1.
Distribution of study participants and demographic characteristics on study entry
| Distribution | Group 1 (MenACYW-D) | Group 2 (MCC) |
|---|---|---|
| Randomly assigned participants | 61 (100) | 62 (100) |
| Received study vaccine at 12 months | 60 (98) | 62 (100) |
| Received study vaccine at 18 months | 58 (95) | NA |
| Discontinued at any point | ||
| Voluntary withdrawal | 3 (3) | 2 (2) |
| Lost to follow-up | 1 (1) | 0 (0) |
| Protocol noncompliance | 0 (0) | 2 (2) |
| Completed study | 57 (93) | 58 (94) |
| Full analysis set | 55 (90) | 62 (100) |
| DTaP-IPV-Hib full analysis set | 51 (84) | NA |
| Safety analysis set | 60 (98) | 62 (100) |
| Demographic characteristic | Group 1 (n=61) | Group 2 (n=62) |
|
| ||
| Female sex, % | 56 | 53 |
| Median age, months (minimum, maximum) | 12.4 (12.0, 13.2) | 12.3 (12.0, 13.1) |
Data presented as n (%) unless otherwise indicated. DTaP-IPV-Hib Diphtheria, tetanus, pertussis (acellular component), poliomyelitis (inactivated) and Haemophilus type b conjugate vaccine (adsorbed); MenACYW-D Quadrivalent meningococcal conjugate vaccine; MCC Meningococcal conjugate group C vaccine; n Values based on all participants available at enrollment; NA Not applicable;
Antibody titres
At 18 months of age, six months after receiving the first MenACYW-D dose, protective SBA-BR titres in group 1 were observed: 34.6% for serogroup A, 26.9% for serogroup C, 82.7% for serogroup Y and 71.2% for serogroup W. After the second dose of MenACYW-D, a robust antibody response was observed against all four antigens (100% for serogroup A, 96.1% for serogroup C, 100% for serogroup Y and 98% for serogroup W) (Table 2). For group 2, protection against sero-group C was approximately 67% one month postvaccination and declined to 26% at seven months postvaccination. This value was similar to the 18-month pre-dose 2 level of group 1 (Table 2).
TABLE 2.
Percent of patients with protective (≥8[1 / dilution]) antimeningococcal antibody titres (full analysis set)
| Serogroup | Group 1 (n=52) before 18-month MenACYW-D | Group 1 (n=49 to n=51)* one month after 18–month MenACYW-D | Group 2 (n=60) one month after 12-month MCC | Group 2 (n=58) seven months after 12-month MCC |
|---|---|---|---|---|
| A | 34.6 (22.0–49.1) | 100 (93–100) | 1.7 (0.04–8.9) | 3.4 (0.4–11.9) |
| C | 26.9 (15.6–41.0) | 96.1 (86.5–99.5) | 66.7 (53.3–78.3) | 25.9 (15.3–39.0) |
| Y | 82.7 (69.7–91.8) | 100 (93–100) | 60 (46.5–72.4) | 67.2 (53.7–79.0) |
| W | 71.2 (56.9–82.9) | 98 (89.6–100) | 8.3 (2.8–18.4) | 19 (9.9–31.4) |
Data presented as % (95% CI). Antibody titres determined by serum bactericidal assay in the presence of baby rabbit complement.
Sample size varies depending on available data; MenACYW-D Quadrivalent meningococcal conjugate vaccine; MCC Meningococcal conjugate group C vaccine; n Values based on all participants available
Antibody concentrations and titres elicited by DTaP-IPV-Hib components did not appear to be adversely affected by MenACYW-D coadministration. The seroprotection rates and booster response rates were generally similar for groups 1 and group 2, although there was a tendency for group 2 to have lower booster response rates (Table 3).
TABLE 3.
Percent of patients achieving seroprotection (seroconversion for pertussis antigens) by DTaP-IPV-Hib one month postvaccination (DTaP-IPV-Hib full analysis set)
| Group 1 (MenACYW-D) (n=35 to 53)* | Group 2 (MCC) (n=41 to 54) | Historical controls (n=178 to 218)† | |
|---|---|---|---|
| Diphtheria (≥0.1 IU/mL) | 100 (92.3–100) | 100 (93.4–100) | 99.1 (96.7–99.9) |
| Tetanus (≥0.1 IU/mL) | 100 (92.5–100) | 100 (93.3–100) | 100 (98.3–100) |
| Pertussis booster response‡ | |||
| PT* | 100 (92.0–100) | 94.3 (84.3–98.8) | 96.7 (93.4–98.7) |
| FHA | 97.7 (88.0–99.9) | 88.0 (75.7–95.5) | 83.2 (77.5–87.9) |
| PRN | 92.7 (80.1–98.5) | 78.8 (65.3–88.9) | 86.9 (81.6–91.1) |
| FIM | 100 (91.6–100) | 96.8 (85.7–99.5) | 95.7 (92.1–98.0) |
| PRP-T (≥1 ug/mL) | 100 (92.3–100) | 100 (93.3–100) | 99.1 (96.8–99.9) |
| Poliovirus (≥8 1/dilution) | |||
| Type 1 | 100 (92.3–100) | 100 (92.3–100) | 99.5 (97.5–100) |
| Type 2 | 100 (92.3–100) | 100 (92.7–100) | 99.5 (97.5–100) |
| Type 3 | 100 (92.1–100) | 100 (92.7–100) | 99.5 (97.5–100) |
Data presented as % (95% CI).
Sample size varies depending on usable data;
Data from Grimprel et al (15);
≥2-4-fold (dependent on predose titre) increase in titre. DTaP-IPV-Hib Diphtheria, tetanus, pertussis (acellular component), poliomyelitis (inactivated) and Haemophilus type b conjugate vaccine (adsorbed); FHA Filamentous hemagglutinin; FIM Fimbriae; MenACYW-D Quadrivalent meningococcal conjugate vaccine; MCC Meningococcal conjugate serogroup C vaccine; n Values based on all participants available; PRN Pertactin; PRP-T Polyribosylribitol phosphate conjugated to tetanus toxoid; PT Pertussis toxoid
At 18 months of age (six months after receiving the first MenACYW-D dose), GMTs (1/dilution) were 15.8 for serogroup A, 12.1 for serogroup C, 94.2 for serogroup Y and 61.5 for serogroup W (Table 4). One month after dose 2 of MenACYW-D (at 19 months of age), the serogroup C GMT among group 1 children was 957 (95% CI 594 to 1540). For other serogroups, the GMT responses were of a similar or higher magnitude. In contrast, one month after MCC vaccination, the GMT was 71 (95% CI 39.3 to 128) and seven months after MCC vaccination, the serogroup C GMT had declined to 11 (95% CI 6.8 to 18.1), similar to the GMTs pre-dose 2 for the MenACYW-D group. Dose 2 MenACYW-D GMTs against other serogroups were higher among group 1 vaccinees than among those in group 2 at the same time assessment (A: 1740 versus 4.4; Y: 719 versus 31.3; W: 970 versus 5.3). MCC did not contain antigens against these sero-groups (Tables 2 and 4).
TABLE 4.
Geometric mean titre against meningococcal serogroups (full analysis set)
| Serogroup | Group 1 (n=52) before 18-month MenACYW-D | Group 1 (n=51) one month after 18-month MenACYW-D | Group 2 (n=60) one month after 12-month MCC | Group 2 (n=58) seven months after 12-month MCC |
|---|---|---|---|---|
| A | 15.8 (8.7–28.6) | 1740 (1301–2327) | 4.3 (3.7–5.1) | 4.4 (3.8–5.0) |
| C | 12.1 (7.0–20.9) | 957 (594–1540) | 71 (39.3–128) | 11 (6.8–18.1) |
| Y | 94.2 (59.1–150) | 719 (553–935) | 31.3 (19.6–50) | 67.1 (38.4–117) |
| W | 61.5 (35.5–107) | 970 (686–1371) | 5.3 (4.1–6.7) | 7.4 (5.2–10.3) |
Data presented as titre (95% CI). Geometric mean titre determined by serum bactericidal assay in the presence of baby rabbit complement; all results are in units of 1/dilution. MenACYW-D Quadrivalent meningococcal conjugate vaccine; MCC Meningococcal conjugate serogroup C vaccine; n Values based on all participants available
Safety data
Table 5 summarizes the safety profile for each group and dose. Injection site reactions tended to be reported more frequently after dose 2 of MenACYW-D, while systemic solicited reactions were similar for both doses of MenACYW-D and in both groups (Figure 1). Redness and tenderness were the most commonly reported injection-site reactions, whereas irritability was the most frequently reported solicited systemic reaction. Grade 3 reactions were uncommon, and the most frequently observed grade 3 solicited reactions were fever (5.2%), irritability (3.4%) and tenderness (3.4%) (Figure 1).
TABLE 5.
Overview of safety data after each and any meningococcal dose
| Participants with ≥1: | Group 1: Dose 1 (n=60) | Group 1: Dose 2 (n=58) | Group 2 (n=62) |
|---|---|---|---|
| Immediate adverse event | 0 (0–6.0) | 0 (0–6.2) | 1.6 (0–8.7) |
| Solicited adverse reaction | 90.0 (79.5–96.2) | 93.1 (83.3–98.1) | 91.7 (81.6–97.2) |
| Injection site adverse reaction | 55.0 (41.6–67.9) | 67.2 (53.7–79.0) | 55.0 (41.6–67.9) |
| Systemic adverse reaction | 88.3 (77.4–95.2) | 84.5 (72.6–92.7) | 80.0 (67.7–89.2) |
| Unsolicited adverse event | 71.7 (58.6–82.5) | 55.2 (41.6–68.3) | 59.7 (46.4–71.9) |
| Unsolicited adverse reaction | 5.0 (1.0–13.9) | 0 (0–6.2) | 4.8 (1.0–13.5) |
| Serious adverse event | 1.7 (0–8.9) | 0 (0–6.2) | 3.2 (0.4–11.2) |
| Adverse event-related withdrawal | 0 (0–6.0) | 0 (0–6.2) | 0 (0–5.8) |
Data presented as % (95% CI). n Values based on all participants available
Figure 1).
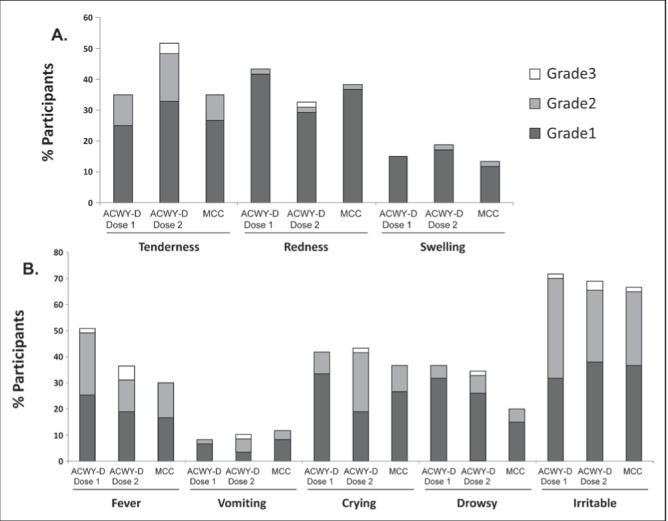
Frequency of solicited adverse reactions according to study group and intensity. ACWY-D Quadrivalent meningococcal conjugate vaccine; MCC Meningococcal conjugate group C vaccine
Three serious adverse events were reported, and none were considered to be vaccination-related by investigators (febrile convulsion in group 1 106 days after dose 1 of MenACWY-D, PCV13 and MMRV vaccines; bronchiolitis in group 2 188 days after MCC, PCV13 and MMRV doses, and 14 days after the DTaP-IPV-Hib and MMR doses; rickets in group 2 126 days after MCC, PCV13 and MMRV doses). There were no deaths during the study, and all participants completed the trial.
DISCUSSION
The present study described the safety and immunogenicity of MenACYW-D administered at 12 and 18 months of age versus a single dose of MCC at 12 months of age, both coadministered with routine childhood vaccinations. Current immunizations programs in Canada use quadrivalent meningococcal conjugate vaccines or MCC vaccines for adolescents and MCC for infants up to 12 months of age. Routine use of quadrivalent meningococcal conjugate vaccine in toddlers could expand protection against IMD caused by A, C, Y and W serogroups; this could lead to expanded meningococcal disease prevention throughout childhood. The study results demonstrate that MenACYW-D has a good immunogenicity and safety profile, and can be used as an alternative to MCC in Canadian vaccination programs. In particular, we found that the second dose of MenACYW-D resulted in high seroprotection rates against four serogroups and potentially better protected toddlers from IMD. In the other study arm, sero-group C seroprotection rates had declined to 26% seven months after a single dose of MCC vaccine, similar to the seroprotection rate for serogroup C six months after the first dose of MenACYW-D.
A second study objective was to describe the immunogenicity of components of routine combination vaccines administered at 12 and 18 months age when administered concurrently with MenACYW-D. Antibody responses to all DTaP-IPV-Hib antigens were generally similar for both groups. Furthermore, these immunogenicity results were similar to those of a historical control (Table 5) (15). Therefore, a clinically important interference between MenACYW-D and DTaPIPV-Hib at 18 months of age is unlikely.
One limitation of the present study is that antibody responses and interactions among MenACYW-D, PCV13 and MMRV at the 12-month dose, and between MenACYW-D and MMR at the 18-month dose were not examined. Such comparisons were not possible because of the limited blood volume and frequency of collection. However, Pina et al (14) found no negative interference when MenACYW-D was administered as a second dose at 12 months of age with MMRV. When PCV7 was coadministered with MenACYW-D, >98% of recipients achieved seroprotection to all vaccine serogroups, although GMTs tended to be lower (14).
Data regarding duration of the antibody responses after a two-dose series of MenACYW-D are not available for the 12- and 18-month two-dose schedule. Similarly, the potential for a booster effect has yet to be evaluated among adolescents who received a quadrivalent conjugate vaccine in early childhood.
The safety profiles of both schedules were good and generally similar. While some injection site and systemic reactions were more frequently reported in group 1 than in group 2, grade 3 reactions were infrequent and there were no withdrawals as a result of safety concerns.
Overall, a two-dose schedule of MenACYW-D resulted in higher bactericidal antibody responses at 19 months of age against serogroup C than one MCC dose alone (Table 4) and also provided high responses against serogroups A, Y and W.
Data describing MenACYW-D use in infant and toddlers first began to appear in 2006 (16) and more fully in Pina et al (14) in 2012. The data presented in the present study extend and confirm the findings of these previous studies. Our data indicate that a 12- and 18-month schedule may be an alternative to MCC at 12 months of age, the current routine in most Canadian provinces, should broader serotype protection become desirable. Additional clinical research should be conducted to further explore the best and most cost-effective methods to prevent meningococcal disease in Canada.
Acknowledgments
This study was funded by Sanofi Pasteur. The authors acknowledge the work of John Bukowski PhD of Words World Consulting and Ronald Gold MD, who wrote a first draft of the manuscript under the supervision of the authors. They were paid for their work by Sanofi Pasteur. The authors also acknowledge the work of Robert Lersch PhD of Sanofi Pasteur who coordinated the writing of the manuscript, as well as reviewed and edited the manuscript. The authors wish to declare the following conflicts of interest: Francisco Noya received honoraria and travel expenses to present these data at the Asociación Mexicana de Infectologia Pediátrica in Aguascalientes, Mexico in 2012, and the 31st Annual Meeting of the European Society of Paediatric Infectious Disease (ESPID) in Milan, Italy in 2013. He is also an ad hoc consultant for Pediapharm on asthma products. The Montreal University Health Centre Research Institute received an operational research grant to conduct this study. Donna Reynolds was an employee of Sanofi Pasteur at the time the study commenced and is now an adjunct professor at the University of Toronto. Dion Neame and Philipp Oster are employees of Sanofi Pasteur.
Footnotes
DISCLOSURES: The study originated with and was funded by Sanofi Pasteur. This work was presented in part at the Association of Mexican Pediatric Infectology, November 28 to December 1, 2012, Aguascalientes, Mexico. This work was also presented in part at the European Society for Paediatric Infectious Diseases, May 28 to June 1, 2013, Milan, Italy.
REFERENCES
- 1.Manchanda V, Gupta S, Bhalla P. Meningococcal disease: History, epidemiology, pathogenesis, clinical manifestations, diagnosis, antimicrobial susceptibility and prevention. Indian J Med Microbiol. 2006;24:7–19. doi: 10.4103/0255-0857.19888. [DOI] [PubMed] [Google Scholar]
- 2.Harrison OB, Claus H, Jiang Y, et al. Description and nomenclature of Neisseria meningitidis capsule locus. Emerg Infect Dis. 2013;19:566–73. doi: 10.3201/eid1904.111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pelton SI, Gilmet GP. Expanding prevention of invasive meningococcal disease. Expert Rev Vaccines. 2009;8:717–27. doi: 10.1586/erv.09.37. [DOI] [PubMed] [Google Scholar]
- 4.Kirsch EA, Barton RP, Kitchen L, Giroir BP. Pathophysiology, treatment and outcome of meningococcemia: A review and recent experience. Pediatr Infect Dis J. 1996;15:967–78. doi: 10.1097/00006454-199611000-00009. quiz 79. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2005;54(RR-7):1–21. [PubMed] [Google Scholar]
- 6.Granoff DM, Pelton S, Harrison D. Meningococcal vaccines. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 6th edn. Philadelphia: Saunders Elsevier; 2012. pp. 388–418. [Google Scholar]
- 7.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27(Suppl 2):B51–63. doi: 10.1016/j.vaccine.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 8. Health Canada Web Site. Drugs and Health Products: Bexsero. 2014. < www.hc-sc.gc.ca/dhp-mps/prodpharma/sbd-smd/drug-med/sbd_smd_2014_bexsero_147275-eng.php> (Accessed February 26, 2014)
- 9.National Advisory Committee on Immunization (NACI) An update on the invasive meningococcal disease and meningococcal vaccine conjugate recommendations. An Advisory Committee Statement (ACS) Can Commun Dis Rep. 2009;35(ACS-3):1–40. [PubMed] [Google Scholar]
- 10.Public Health Agency of Canada (PHAC) Invasive meningococcal disease (IMD) 2012. < www.phac-aspc.gc.ca/im/vpd-mev/meningococcal-eng.php> (Accessed July 2, 2013)
- 11.Wilder-Smith A, Goh KT, Barkham T, Paton NI. Hajj-associated outbreak strain of Neisseria meningitidis serogroup W135: Estimates of the attack rate in a defined population and the risk of invasive disease developing in carriers. Clin Infect Dis. 2003;36:679–83. doi: 10.1086/367858. [DOI] [PubMed] [Google Scholar]
- 12.Public Health Agency of Canada (PHAC) Publicly funded immunization programs in Canada – routine schedule for infants and children including special programs and catch-up programs (as of March 2013) 2013. < www.phac-aspc.gc.ca/im/ptimprogprogimpt/table-1-eng.php> (Accessed July 2, 2013)
- 13.U.S. National Institutes of Health. Study of two doses of Menactra® or one dose of monovalent meningococcal group C vaccine with routine immunizations. 2013. < http://clinicaltrials.gov/ct2/show/NCT01359449?term=MTA73&rank=1> (Accessed July 2, 2013)
- 14.Pina LM, Bassily E, Machmer A, Hou V, Reinhardt A. Safety and immunogenicity of a quadrivalent meningococcal polysaccharide diphtheria toxoid conjugate vaccine in infants and toddlers: Three multicenter phase III studies. Pediatr Infect Dis J. 2012;31:1173–83. doi: 10.1097/INF.0b013e318268dfe4. [DOI] [PubMed] [Google Scholar]
- 15.Grimprel E, Wysocki J, Boisnard F, Thomas S, Mwawasi G, Reynolds D. Immunogenicity and safety of fully liquid DTaP(5)-IPV-Hib compared with DTaP(3)-IPV/Hib when both coadministered with a heptavalent pneumococcal conjugate vaccine (PCV7) at 2, 3, 4, and 12 to 18 months of age: A phase III, single-blind, randomised, controlled, multicentre study. Vaccine. 2011;29:7370–8. doi: 10.1016/j.vaccine.2011.07.078. [DOI] [PubMed] [Google Scholar]
- 16.Keyserling HL, Pina M, Hou V, Bassily E, DeTora L, Reinhardt A. Immunogenicity of a quadrivalent meningococcal polysaccharide diphtheria toxoid conjugate vaccine against serogroups A, C, Y, and W-135 (MCV-4) in 9- to 15-month olds. Abstract 640. 44th Annual Meeting of the Infectious Disease Society of America (IDSA); Toronto, Ontario. October 12 to 15, 2006. [Google Scholar]


