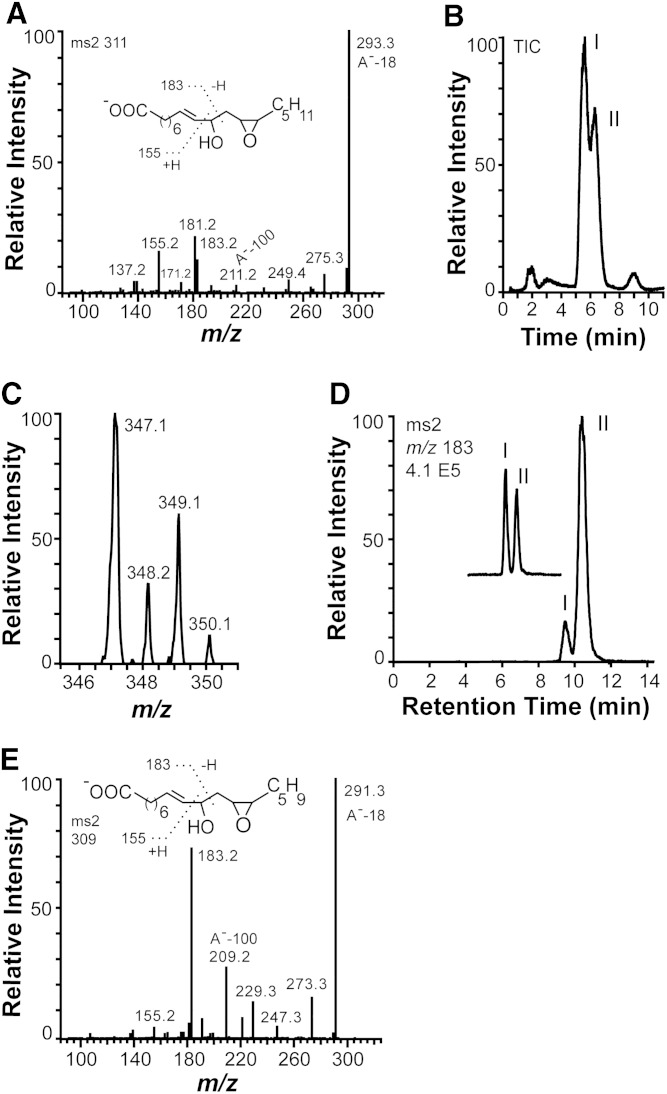
Fig. 3.
MS2 spectra of the epoxy alcohols formed from 18:2n-6 and 18:3n-3 by recombinant 10R-DOX-EAS, analysis of chlorohydrin derivatives, and separation of isotopes and stereoisomers. A: MS2 spectrum of the epoxy alcohol [12S(13R)-epoxy-10(R)-hydroxy-8(E)-octadecenoic acid] formed from 18:2n-6. B, C: LC/MS analysis (m/z 347–349 full scan) of chlorohydrin derivatives, which were obtained by treatment of products formed from 18:2n-6 by recombinant MGG_10589 with methoxime HCl. The two main peaks, I and II, in B yielded the same MS2 and MS3 spectra. The molecular anions at m/z 347 and 349 with incorporation of one chlorine atom (35Cl or 37Cl). D: Products formed during oxidation of 12R(13S)-epoxy-(9Z)-octadecenoic acid by MGG_10859 and separation of the syn and anti isomers of 12R(13S)-epoxy-10-hydroxy-8(E)-octadecenoic acid by NP-HPLC (peaks marked I and II). The partial chromatogram of the inset was obtained after addition of the epoxy alcohol formed from 18:2n-6 by MGG_10859 to the products formed from 12R(13S)-epoxy-(9Z)-octadecenoic acid, illustrating that biological product formed from 18:n-6 coelutes with the first eluting stereoisomer in peak I [syn; 12S(13R)-epoxy-10(R)-hydroxy-8(E)-octadecenoic acid; see text for details]. E: MS2 spectrum of 12(13)-epoxy-10(R)-hydroxy-8(E),15(Z)-octadecenoic acid formed from 18:3n-3 by 10R-DOX-EAS.