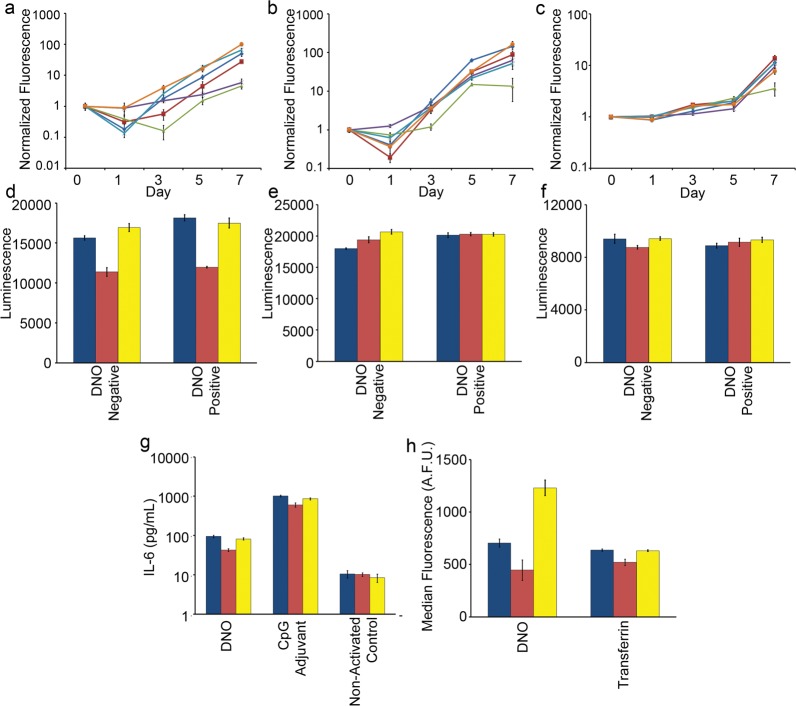
Figure 3.
Measuring the impact of medium modifications on cell growth, viability and phenotype. (a) Mouse 3T3 fibroblasts, (b) human HEK-293 embryonic kidney, and (c) human H441 adenocarcinoma cell lines were seeded into standard medium (dark blue line), Mg2+-adjusted (red line), heat-inactivated FBS (green line), Mg2+ + heat-inactivated FBS (purple line), 200 nM actin (light blue line), or combination Mg2+ + 200 nM actin medium (orange line). Change in cell number was estimated by fluorescence of CyQuant stain over time and was normalized to signal on day 0. Cell viability and metabolism of (d) 3T3, (e) HEK-293, and (f) H441 cells were profiled after 24 h incubation with and without 5 nM DNO. (g) Primary mouse immune cells were isolated from spleens and incubated with 1 nM DNO, an equivalent mass of CpG phosphorothioate oligonucleotide adjuvant, or medium only, and the concentration of IL-6 cytokine released into the supernatant was determined by ELISA. (h) 3T3 fibroblast cells were incubated for 16 h with 1 nM fluorescently labeled DNO or transferrin, and endocytosis of the two agents was measured by flow cytometry after extensive washing to remove surface-bound particles. (d–h) Culture conditions are standard medium (blue), medium + 6 mM Mg2+ + heat-inactivated FBS (red), or medium + 6 mM Mg2+ + FBS + 200 nM actin (yellow). (a–h) Error bars represent standard error of the mean of n = 5 replicates.