Abstract
Background:
Basic fibroblast growth factors (bFGFs) play a crucial role in wound healing by promoting fibroblast proliferation and neovascularization. However, drawback of bFGF is short half-life in free form. Gelatin has a capability of sustaining growth factors, which are gradually released while degradation. The purpose of this study is to see whether bFGF-impregnated gelatin sheet is effective in a murine model and whether it could also be available for patients in a safe manner.
Methods:
Full-thickness skin defect was created on C57BL/6J mice and covered with bFGF with gelatin sheet (group A), bFGF without gelatin sheet (group B), phosphate buffer saline (PBS) with gelatin sheet (group C), and only PBS (group D). Wound healing was evaluated in terms of percent wound closure, granulation thickness, wound maturity, and vascular density. Clinical trial was conducted for patients who received either acute or chronic ulcers. The sheets were put onto the wounds and covered by hydrocolloid dressing, which was changed weekly.
Results:
Groups A and B exhibited better wound healing than groups C and D in all aspects. Moreover, group A showed better results than group B at day 7 in terms of wound closure, collagen maturity, and vascularity. Efficacy without any adverse events was found in the clinical series.
Conclusions:
These findings suggest that control-released bFGF using gelatin sheet is effective for promoting wound healing. Such therapeutic strategy was considered to offer several clinical advantages including rapid healing and reduction of the dressing change with less patient discomfort.
High incidence of chronic wound including pressure ulcers and lower leg ulcers due to diabetes, ischemia, and so on represents a significant problem with few solutions. Particularly, in developed countries, it has been estimated that 1–2% of the population will experience a chronic wound during their lifetime and the associated costs are estimated to 2–4% of the total health care expenses.1 For such wounds, various therapeutic approaches have been developed based on the concept of regeneration medicine such as cell-based therapy and growth factor-based therapy.2–4
The fibroblast growth factors (FGFs) are a family of polypeptide that is mitogenic for a broad range of cell types and mediators of a wide spectrum of developmental and pathophysiological processes in vivo and in vitro.5,6 The basic FGF (bFGF), which is one of the 22 different isotypes of FGF, plays a crucial role in wound healing process by promoting fibroblast proliferation, inducing neovascularization, and increasing the synthesis of collagenase.7–10 As human recombinant bFGF has been commercially available in Japan, topical administration of bFGF has shown to be effective for wound healing in clinical situations.11,12 However, daily administration of bFGF is required for wound healing due to its short half-life in vivo, which occasionally is time-consuming and painful and/or results in discomfort for patients and a potential risk of infection.
Gelatin is a denatured extract of collagen and has a biodegradable property. Recently, Tabata and coworkers13,14 have demonstrated a novel approach with a drug delivery system using gelatin that enabled controlled release of bFGF while it undergoes hydrolysis in vivo and thus improved efficacy of growth factor therapy. Thereafter, several preclinical and clinical studies have been conducted and shown that such control-released bFGFs contribute to healing process of many tissues and neovascularization.15–24 Therefore, the purpose of this study is to see whether bFGF-impregnated gelatin sheet is effective for wound healing compared with the conventional spraying administration in a murine model. In addition, we investigated the safety of such materials for the treatment of patients with skin ulcers.
MATERIALS AND METHODS
bFGF-Impregnated Gelatin Sheet
All the steps of gelatin sheet production that are briefly described below were carried out under the good manufacturing product-leveled clean condition. Gelatin sheet was prepared by cross-linking with glutaraldehyde. Briefly, 5 wt% aqueous solution of gelatin containing 0.05 wt% glutaraldehyde was flown into the container of the polypropylene (14 × 14 cm2) for 12 hours at 4°C as previously described.14,24 Following the cross-linking reaction, the resulting sheet of gelatin hydrogel was dissected into 2 × 2 cm pieces. Following the freeze-drying step, the sheet was sterilized with ethylene oxide gas. A bFGF that was manufactured from the solution of human recombinant bFGF with an isoelectric point of 9.6 (10 mg/ml) was supplied by Kaken Pharmaceutical (Tokyo, Japan). Before use, freeze-dried gelatin sheet was immersed with aqueous solution of bFGF at the concentration of 7 µg per cm2, which was referred to as bFGF-impregnated gelatin sheet.
Mouse Wound Model
C57BL/6J male mice aged 8 weeks were purchased from Sankyo, Japan. These mice were shaved and depilated under anesthesia with avertin (2, 2, 2, tribromoethanol, 2-methyl-2-butanol). Stented, cutaneous wounds were created as previously described.25,26 Briefly, bilateral 6-mm full-thickness wounds were excised on the dorsum by punch biopsies. India ink was applied intradermally to mark the wound margins. A silicone stent with an 8-mm inner diameter was sutured with 5-0 nylon (Ethicon) around each wound to minimize skin contracture and ensure healing by secondary intention. Immediately after wounding, the following treatment group was applied topically on the wound: group A: bFGF-impregnated gelatin sheet (7 µg/cm2); group B: conventional method of daily bFGF spray (1 µg/cm2/d) without gelatin sheet; group C: PBS with gelatin sheet; and group D: PBS without gelatin sheet. Film dressing was covered to prevent drying and contamination. Gelatin sheets in groups A and C were changed weekly.
Wound Closure by the Macroscopical Analyses
Photographs of the wound were taken at postoperative days 0, 3, 5, 7, 10, and 14 followed by evaluation with computer software BZ-2 Analyzer, Exe 1.41 (Keyence, Japan). The area of skin defect and the inner circle of the stent were measured at each time point. The skin defect area/inner stent area ratio on day 0 was determined as control (100%) for the ratios of later observation on days 3, 5, 7, 10, and 14.25
Morphological Studies
The wounds were harvested from euthanized animals at postoperative days 3, 5, 7, 10, and 14 (n = 4 per group at each time point). The harvested wounds were fixed with 100% methanol and embedded in paraffin. Sections were cut from the central region of the wound at a thickness of 5 μm. Before staining, paraffin sections were deparaffinized and rehydrated by successive passages through xylene. Hematoxylin and eosin staining was performed to observe granulation thickness from edge to edge of the ulcer. Sections were imaged and digitized in their entirety at the 200-fold magnification (200×) with BZ-9000 (Keyence, Japan).
Van Gieson’s Stain for Wound Maturity
Wound maturity was semiquantified with Van Gieson’s staining as previously described.27 The staining demonstrates mature collagen in deep red and immature collagen in pink. Four horizontal sections through the center of the entire wound were analyzed per wound at each time point. Sections were imaged and digitized in their entirety at 200× with BZ-9000. Then images were analyzed by 3 blind reviewers with BZ-2 Analyzer, Exe 1.41 (Keyence, Japan). Percentage of mature collagen was quantified by measuring the total pixel area of the wound and dividing it by the percentage of pixels therein that were consistent in color with mature collagen.
CD31 Staining for Vascularity
Paraffin sections were stained with CD31 (Purified Rat anti-mouse CD31, BD) followed by DAB (Vector Laboratories, Burlingame, CA) staining to examine the vascular density of the wound post therapy. Four fields were randomly selected and CD31-DAB positively stained vessels were counted using BZ-2 Analyzer, Exe 1.41 by 3 blind reviewers.
Statistical Analysis
All experimental values were expressed as the mean ± standard deviation. One-way analysis of variance was used for multigroup comparisons. Student’s t test was used for pairwise comparisons. Statistical significance was defined as a P value <0.05.
Clinical Trials
Based on the findings obtained from the preclinical studies, we have conducted phase I clinical trial to ensure the safety of bFGF-impregnated gelatin sheet for acute/chronic wound healing (Effects of gelatin sheet incorporating basic fibroblast growth factor for treatments of skin ulcers, UMIN Clinical Trial Registry at http://www.umin.ac.jp/ctr, Identification number: UMIN000001068).
All patients, who have no history of malignancy in the past 5 years, provided written informed consent to participate in this study, which proceeded according to the ethical rules for human experimentation stated in the 1975 Declaration of Helsinki and under the approval of the Institutional Review Board of Nippon Medical School Hospital (Approval Number: 19-09-29). Surgical debridement for nonviable tissue was carried out under the general anesthesia before the application of bFGF-impregnated gelatin sheet. Such sheet was put onto each wound and fixed with the occlusive hydrocolloid dressing. Dressing change was made weekly until the wounds were either completely epithelialized or covered with granulation tissue on which skin grafting can be made for the maximum of 6 weeks.
RESULTS
bFGF-Impregnated Gelatin Sheet Accelerates Wound Closure
Wounded animals treated with bFGF-impregnated gelatin sheet (group A) showed significantly faster wound closure compared with all other groups at day 7, an early stage in wound healing (group A 44.8% ± 5.2% vs group B 37.3% ± 3.7%, P < 0.05; group C 26.8% ± 5.5%, P < 0.05; group D 26.3% ± 9.3%, P < 0.05). Although the significant difference of wound closure between group A and group B disappeared starting at day 10, the bFGF-treated group demonstrated significantly accelerated wound closure compared with the non–bFGF-treated group. In addition, there was no significant difference in percent wound closure between group C and group D through all time points (Fig. 1).
Fig. 1.
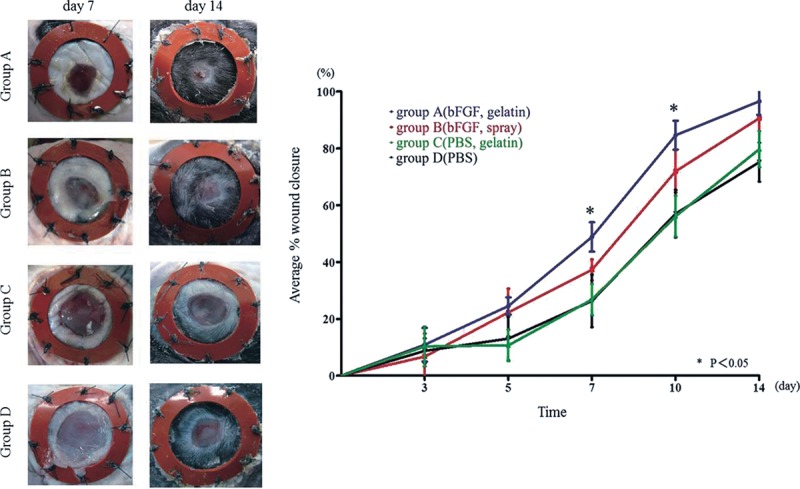
Time course of wound closure. A, Macroscopic photographs of wounds at day 7 and day 14 demonstrate the significantly faster wound closure in group A. B, Average wound closure from day 0 to day 14 [expressed as a percent of the day 0 control (100%)]. At day 7, group A showed a greater extent of wound closure compared with groups B, C, and D. At day 10, the difference between groups A and B disappeared, but there was a significant difference between the bFGF-treated groups and the non–bFGF-treated groups. *P < 0.05.
Groups Treated with bFGF Promotes Granulation Thickness
Groups A and B showed the significantly thicker wound granulation compared with groups C and D from all time points after day 5. The peak of significant difference between group A (day 5: 515 ± 104 µm, day 7: 524 ± 80.4 µm, day 10: 599 ± 67.8 µm, day 14: 629 ± 82.6 µm) and group B (day 5: 519 ± 29.4 µm, day 7: 594 ± 106 µm, day 10: 594 ± 90.7 µm, day 14: 645 ± 93.6 µm) vs group C (day 5: 260 ± 69.2 µm, day 7: 336 ± 49.2 µm, day 10: 418 ± 35.8 µm, day 14: 408 ± 88.0 µm) and group D (day 5: 188 ± 40.7 µm, day 7: 421 ± 52.4 µm, day 10: 461 ± 23.5 µm, day 14: 426 ± 20.6 µm) was seen at each time point (P < 0.05). In groups A and B using bFGF demonstrated similar result, thickness of the granulation increased from day 3 to day 5, and became plateau after day 5. On the other hand, the thickness of the granulation gradually increased with a time course in group C and group D from day 5 (Fig. 2).
Fig. 2.
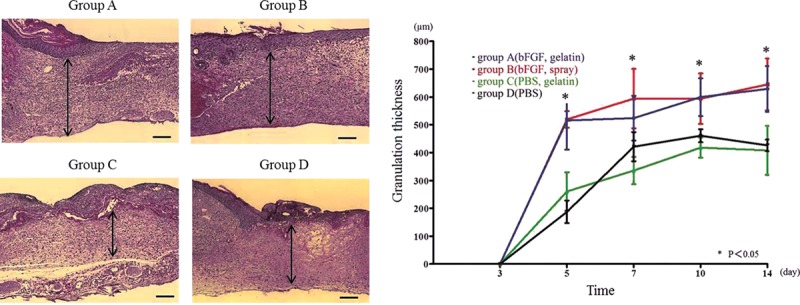
Histological evaluation and quantification of granulation thickness. A, Hematoxylin and eosin staining was performed to observe granulation thickness in each group at day 7. The arrow indicates the thickness of granulation, which was significantly greater in group A. B, Time course of granulation thickness shows a greater thickness in groups A and B compared with groups C and D at day 5, 7, 10, and 14 (bar: 200 µm). *P < 0.05.
bFGF-Impregnated Gelatin Sheet Stimulates Collagen Maturity
The collagen maturity increased in all groups with time following wounding. Similar to wound closure data, group A (631 ± 142.6 pixels/hpf of 200×) demonstrated significantly greater area of mature collagen at early time point of wound healing of day 7 compared with all other groups (group B: 169 ± 127.2 pixels/hpf of 200×, group C: 0 ± 0, group D: 0 ± 0; P < 0.05). Group B following the healing of group A demonstrated similar wound collagen maturity to group A starting at day 10 (7687 ± 1828 pixels/hpf of 200× vs 4259 ± 851.2 pixels/hpf of 200×). Groups C and D, the non–bFGF-treated group, demonstrated significantly smaller area of wound collagen maturation at day 10 compared with groups treated with bFGF (Fig. 3).
Fig. 3.
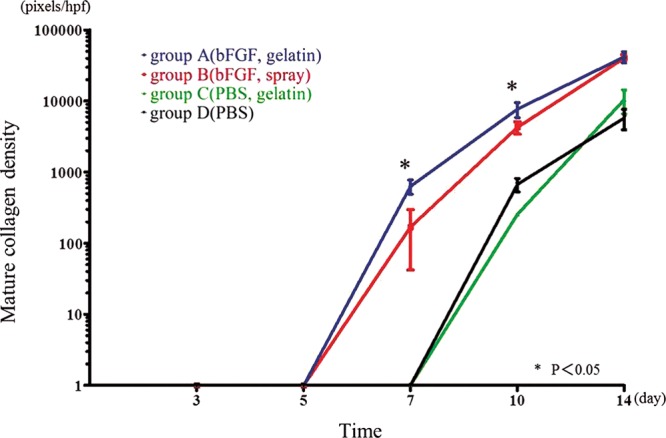
Collagen maturation as determined by Van Gieson’s staining. A higher number of red pixels/hpf is indicative of a higher density of mature collagen. At day 7, group A demonstrated a significantly greater density of mature collagen compared with all other groups. At day 10, group B showed a similar density of mature collagen relative to group A, and this area was significantly greater than that of groups C and D. *P < 0.05.
bFGF-Impregnated Gelatin Sheet Therapy Enhances Wound Vascularization
Similar to the results of wound closure, the wounds treated in group A (day 7: 185.4 ± 21.7 counts/hpf, day 10: 194 ± 22.7 counts/hpf) showed significantly higher number of CD31 counts compared with all other groups at day 7 (P < 0.05) and day 10 (P < 0.05). However, group A and group B demonstrated a decrease in the number of CD31-positive vascular structure at day 14. Although groups C and D exhibited significantly lower wound vascularization at days 7 and 14 than the other groups, the number of vessels gradually increased from day 3 to 14 (Fig. 4).
Fig. 4.
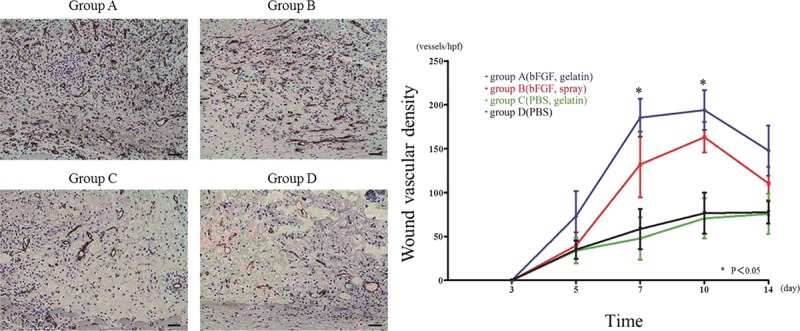
Wound vascularization. A, Anti-CD31 antibody staining at day 7. The brown-colored areas correspond to blood vessels stained by the anti-CD31 antibody. Stronger CD31 staining was observed for group A compared with the other groups, indicative of a larger number of vascular structures. B, Graph demonstrating density (pixels/hpf) of CD31-positive staining over the time course of 3–14 d. Group A showed a significantly higher number of CD31-positive pixels compared with the other groups at day 7 and day 10 (bar: 50 µm).
bFGF-Impregnated Gelatin Sheet Is Effective for Patients with Various Skin Ulcers
Since January 2009, we have treated totally 8 patients who received either acute or chronic ulcers, all of which had the indication for conventional treatments using bFGF, with this protocol (Table 1). The cause of the ulcers included trauma, burn, venous congestion, diabetes, and collagen diseases. We found no adverse events in this series. Complete epithelialization was achieved in 6 patients without any further surgical procedures. Mesh skin grafting was applied 3 weeks after initial application of bFGF-impregnated gelatin sheet in 1 case because the wound was covered by sufficient granulation tissue. Only 1 case did not exhibit complete epithelialization although the size of the ulcer was decreased to approximately 60% of the original size.
Table 1.
Patient Demographics
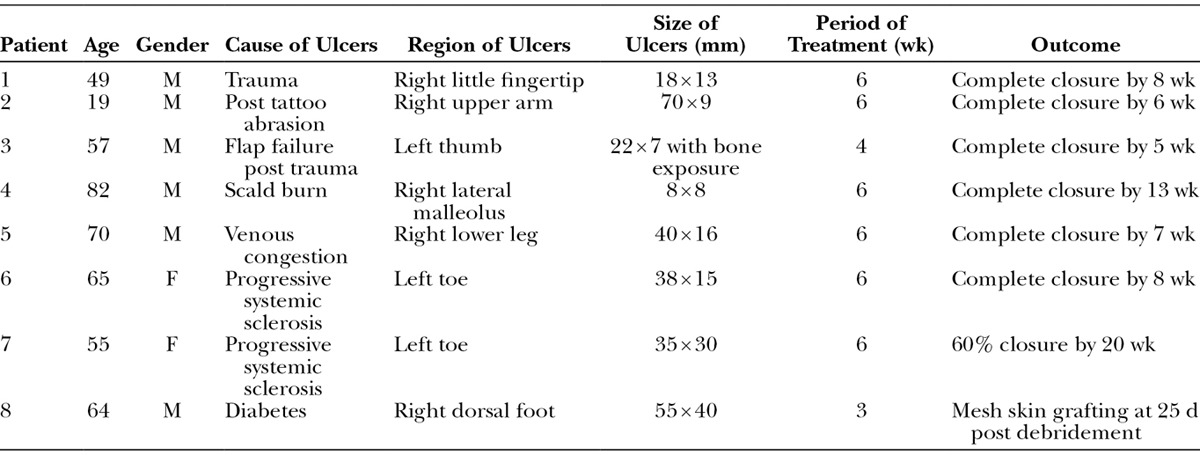
Case 3.
A 57-year-old male presented chronic skin ulcer, which was 22 × 7 mm in size, with pharangeal bone exposure of his left thumb post trauma. Several surgical attempts have been made for the coverage but all have failed for 2 years. After the written consent, skin margin of the ulcer and the superficial layer of the pharangeal bone were debrided under general anesthesia and then covered with bFGF-impregnated gelatin sheet simultaneously, and the dressing was changed weekly. The ulcer was almost closed after 3 weeks and complete healing was achieved by 5 weeks due to wound contraction and epithelialization (Fig. 5).
Fig. 5.
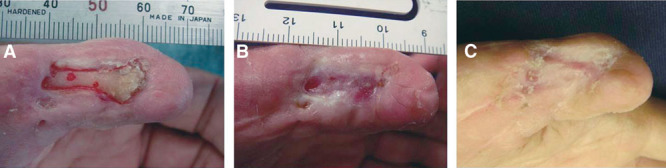
Chronic ulcer with pharangeal bone exposure on the left thumb (case 3). A, Preoperative appearance. Following debridement, a bFGF-impregnated gelatin sheet was applied over the wound. B, Three weeks post application. Most of the wound area was covered by epithelialized tissue. Subsequently, complete wound closure was achieved by 5 wk. C, Appearance at 12 wk post application.
Case 6.
A 65-year-old female presented chronic ulcers, which was 38 × 15 mm in size, post amputation of her left great toe due to gangrene caused by progressive systemic sclerosis. Three days after the debridement under general anesthesia, bFGF-impregnated gelatin sheet was applied onto the wound, and the dressing was changed weekly. Wound was completely epithelialized by 7 weeks without any adverse event (Fig. 6).
Fig. 6.
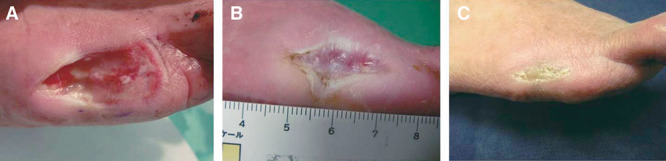
Chronic postamputation ulcer of the great toe due to progressive systemic sclerosis (case 6). A, Appearance 3 d after debridement. A bFGF-impregnated gelatin sheet was subsequently applied. B, Six weeks post application. The wound area was almost completely covered by epithelialization and wound contraction. C, Appearance at 3 mo post application.
DISCUSSION
bFGF is recognized as one of the potent mitogens, regulating proteins that induce the proliferation of a variety of cells, including epithelial and mesenchymal cells.28,29 Particularly, bFGF serves as a key factor to accelerate wound healing by promoting fibroblast proliferation, inducing neovascularization, and increasing the synthesis of collagenase.7–10 As human recombinant bFGF has been commercially available in Japan, topical administration of bFGF has shown to be effective for various wound healing including burn and radiation ulcer.11,12 However, daily spray application is necessary because the half-life of bFGF in free form is known to be less than 1 hour30 and bFGF is easily eliminated from the applied site by diffusion as it is water soluble. Such daily procedure may be time-consuming, may result in patient discomfort, and may increase the potential risk of infection.
Recently, Tabata and coworkers13,14 designed a novel approach with a drug delivery system that enabled controlled release of a single growth factor by using gelatin hydrogel as a vehicle in vivo and thus improved efficacy of growth factor therapy. They have also clearly shown that 125I-labeled bFGF-impregnated gelatin hydrogel injected subcutaneously in murine model were degraded in 2–4 weeks depending on the water content of hydrogels without nonspecific inflammatory reaction and then induced neovascularization around the injected site.14,31–33 Moreover, the safety of gelatin that we used in this study is considered to be relatively high because it has been widely applied in clinical setting.31,32 Based on these scientific evidences, numerous preclinical studies have been conducted for the purpose of bone fracture healing,18 skull regeneration,22,24 healing for sternum dehiscence,17 healing for pancreatic fistula,15 angiogenesis in ischemic flap,16 and skin ulcer.19,21,23 In addition, some clinical trials using control-released bFGF for improvement of chronic limb ischemia with intractable ulcers have been carried out without any adverse events.20,34
As indicated in the previous studies, bFGF with or without control release have demonstrated rapid wound healing and increased granulation thickness and angiogenesis.10,21,35 However, none of the study has compared the efficacy between control-released bFGF and conventional spray method. Previous publications have revealed that the optimal dose of bFGF by topical spray and bFGF impregnated with gelatin is 1 µg/cm2/d and 7 µg/cm2/wk, respectively.36,37 As the gelatin sheet applied in this study almost degrades in 1 week, the daily amount of bFGF seems to be theoretically equivalent between group A and group B. In our study, significantly better findings in group A compared with group B were (1) wound closure rate at day 7, (2) collagen maturity at day 7, and (3) vascular density at day 7 and day 10. Otherwise, there was no significant difference between group A and group B in any parameters although group A is almost superior to group B at any time point in any evaluation. These findings may indicate that bFGF-impregnated gelatin sheet acts positively at early stage of wound healing process. Another finding that should be addressed is that collagen maturity was better in bFGF-treated group (groups A and B) than in non–bFGF-treated group (groups C and D) and that vascular density decreased through day 10 to day 14 in bFGF-treated group (groups A and B). Generally, fibroblasts and capillary that increase in proliferation phase are considered to eventually decrease in remodeling phase in normal wound healing process. Our findings in this study showed similar pattern, suggesting that bFGF-impregnated gelatin sheet accelerates wound healing. Limitation of our preclinical study may include that bFGF-impregnated gelatin sheet was applied to nonimpaired wound healing model. Although we have confirmed that such procedure is estimated to be effective for impaired wound in clinical trial as described below, another experiment has to be conducted using impaired wound healing model to confirm the efficacy at histological and molecular level.
We have conducted phase I clinical trial with bFGF-impregnated gelatin sheet in 8 patients of which 6 exhibited chronic wound (cases 3–8). To our best knowledge, this case series may be a first clinical report with this procedure. All wounds were completely healed by spontaneous epithelialization or secondary skin grafting except 1 case (case 7) that was diagnosed as progressive systemic sclerosis. As we have experimented with only a small size sample, it is mandatory that appropriate candidates have to be explored with this procedure through a large study, such as a randomized clinical trial, in future to confirm the efficacy of control-released bFGF in wound healing. Nevertheless, unlike cell-based therapy, the application of control-released bFGF can be repeatable and well standardized in both amount and quality aspect in a safe fashion.
CONCLUSIONS
In conclusion, control-released bFGF using gelatin sheet is effective for promoting wound healing. As control-released bFGF with gelatin sheet offers clinical benefits such as accelerated healing, reduction of daily dressing change, and minimization of patient discomforts while hopefully reducing treatment costs, we believe that use of this sheet may be an alternative treatment procedure for patients with chronic ulcers.
Footnotes
Presented, in part, at the 55th Annual Meeting of the Plastic Surgery Research Council, 2010, San Francisco, CA.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyamoto M, Yasutake M, Takano H, et al. Therapeutic angiogenesis by autologous bone marrow cell implantation for refractory chronic peripheral arterial disease using assessment of neovascularization by 99mTc-tetrofosmin (TF) perfusion scintigraphy. Cell Transplant. 2004;13:429–437. doi: 10.3727/000000004783983837. [DOI] [PubMed] [Google Scholar]
- 3.Mizuno H, Miyamoto M, Shimamoto M, et al. Therapeutic angiogenesis by autologous bone marrow cell implantation together with allogeneic cultured dermal substitute for intractable ulcers in critical limb ischaemia. J Plast Reconstr Aesthet Surg. 2010;63:1875–1882. doi: 10.1016/j.bjps.2009.11.037. [DOI] [PubMed] [Google Scholar]
- 4.Taniguchi T, Amoh Y, Tanabe K, et al. Treatment of intractable skin ulcers caused by vascular insufficiency with allogeneic cultured dermal substitute: a report of eight cases. J Artif Organs. 2012;15:77–82. doi: 10.1007/s10047-011-0601-9. [DOI] [PubMed] [Google Scholar]
- 5.Armelin HA. Pituitary extracts and steroid hormones in the control of 3T3 cell growth. Proc Natl Acad Sci U S A. 1973;70:2702–2706. doi: 10.1073/pnas.70.9.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gospodarowicz D. Localisation of a fibroblast growth factor and its effect alone and with hydrocortisone on 3T3 cell growth. Nature. 1974;249:123–127. doi: 10.1038/249123a0. [DOI] [PubMed] [Google Scholar]
- 7.Gospodarowicz D. Biological activities of fibroblast growth factors. Ann N Y Acad Sci. 1991;638:1–8. doi: 10.1111/j.1749-6632.1991.tb49012.x. [DOI] [PubMed] [Google Scholar]
- 8.Hayek A, Culler FL, Beattie GM, et al. An in vivo model for study of the angiogenic effects of basic fibroblast growth factor. Biochem Biophys Res Commun. 1987;147:876–880. doi: 10.1016/0006-291x(87)91011-4. [DOI] [PubMed] [Google Scholar]
- 9.Tassi E, Al-Attar A, Aigner A, et al. Enhancement of fibroblast growth factor (FGF) activity by an FGF-binding protein. J Biol Chem. 2001;276:40247–40253. doi: 10.1074/jbc.M104933200. [DOI] [PubMed] [Google Scholar]
- 10.Tsuboi R, Rifkin DB. Recombinant basic fibroblast growth factor stimulates wound healing in healing-impaired db/db mice. J Exp Med. 1990;172:245–251. doi: 10.1084/jem.172.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akita S, Akino K, Imaizumi T, et al. A basic fibroblast growth factor improved the quality of skin grafting in burn patients. Burns. 2005;31:855–858. doi: 10.1016/j.burns.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Kinoshita N, Tsuda M, Hamuy R, et al. The usefulness of basic fibroblast growth factor for radiation-exposed tissue. Wound Repair Regen. 2012;20:91–102. doi: 10.1111/j.1524-475X.2011.00758.x. [DOI] [PubMed] [Google Scholar]
- 13.Tabata Y, Ikada Y. Vascularization effect of basic fibroblast growth factor released from gelatin hydrogels with different biodegradabilities. Biomaterials. 1999;20:2169–2175. doi: 10.1016/s0142-9612(99)00121-0. [DOI] [PubMed] [Google Scholar]
- 14.Tabata Y, Nagano A, Ikada Y. Biodegradation of hydrogel carrier incorporating fibroblast growth factor. Tissue Eng. 1999;5:127–138. doi: 10.1089/ten.1999.5.127. [DOI] [PubMed] [Google Scholar]
- 15.Aimoto T, Uchida E, Matsushita A, et al. Controlled release of basic fibroblast growth factor promotes healing of the pancreaticojejunal anastomosis: a novel approach toward zero pancreatic fistula. Surgery. 2007;142:734–740. doi: 10.1016/j.surg.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Fujihara Y, Koyama H, Ohba M, et al. Controlled delivery of bFGF to recipient bed enhances the vascularization and viability of an ischemic skin flap. Wound Repair Regen. 2008;16:125–131. doi: 10.1111/j.1524-475X.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- 17.Iwakura A, Tabata Y, Koyama T, et al. Gelatin sheet incorporating basic fibroblast growth factor enhances sternal healing after harvesting bilateral internal thoracic arteries. J Thorac Cardiovasc Surg. 2003;126:1113–1120. doi: 10.1016/s0022-5223(03)00025-4. [DOI] [PubMed] [Google Scholar]
- 18.Kawaguchi H, Nakamura K, Tabata Y, et al. Acceleration of fracture healing in nonhuman primates by fibroblast growth factor-2. J Clin Endocrinol Metab. 2001;86:875–880. doi: 10.1210/jcem.86.2.7199. [DOI] [PubMed] [Google Scholar]
- 19.Kawai K, Suzuki S, Tabata Y, et al. Accelerated wound healing through the incorporation of basic fibroblast growth factor-impregnated gelatin microspheres into artificial dermis using a pressure-induced decubitus ulcer model in genetically diabetic mice. Br J Plast Surg. 2005;58:1115–1123. doi: 10.1016/j.bjps.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Takagi G, Miyamoto M, Tara S, et al. Controlled-release basic fibroblast growth factor for peripheral artery disease: comparison with autologous bone marrow-derived stem cell transfer. Tissue Eng Part A. 2011;17:2787–2794. doi: 10.1089/ten.tea.2010.0525. [DOI] [PubMed] [Google Scholar]
- 21.Kawai K, Suzuki S, Tabata Y, et al. Accelerated tissue regeneration through incorporation of basic fibroblast growth factor-impregnated gelatin microspheres into artificial dermis. Biomaterials. 2000;21:489–499. doi: 10.1016/s0142-9612(99)00207-0. [DOI] [PubMed] [Google Scholar]
- 22.Tabata Y, Yamada K, Hong L, et al. Skull bone regeneration in primates in response to basic fibroblast growth factor. J Neurosurg. 1999;91:851–856. doi: 10.3171/jns.1999.91.5.0851. [DOI] [PubMed] [Google Scholar]
- 23.Takemoto S, Morimoto N, Kimura Y, et al. Preparation of collagen/gelatin sponge scaffold for sustained release of bFGF. Tissue Eng Part A. 2008;14:1629–1638. doi: 10.1089/ten.tea.2007.0215. [DOI] [PubMed] [Google Scholar]
- 24.Yamada K, Tabata Y, Yamamoto K, et al. Potential efficacy of basic fibroblast growth factor incorporated in biodegradable hydrogels for skull bone regeneration. J Neurosurg. 1997;86:871–875. doi: 10.3171/jns.1997.86.5.0871. [DOI] [PubMed] [Google Scholar]
- 25.Fukui T, Kawaguchi AT, Takekoshi S, et al. Liposome-encapsulated hemoglobin accelerates skin wound healing in mice. Artif Organs. 2012;36:161–169. doi: 10.1111/j.1525-1594.2011.01371.x. [DOI] [PubMed] [Google Scholar]
- 26.Galiano RD, Michaels J, V, Dobryansky M, et al. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004;12:485–492. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- 27.Kapoor M, Liu S, Shi-wen X, et al. GSK-3beta in mouse fibroblasts controls wound healing and fibrosis through an endothelin-1-dependent mechanism. J Clin Invest. 2008;118:3279–3290. doi: 10.1172/JCI35381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Rifkin DB, Moscatelli D. Recent developments in the cell biology of basic fibroblast growth factor. J Cell Biol. 1989;109:1–6. doi: 10.1083/jcb.109.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas KA. Fibroblast growth factors. FASEB J. 1987;1:434–440. doi: 10.1096/fasebj.1.6.3315806. [DOI] [PubMed] [Google Scholar]
- 30.Edelman ER, Nugent MA, Karnovsky MJ. Perivascular and intravenous administration of basic fibroblast growth factor: vascular and solid organ deposition. Proc Natl Acad Sci U S A. 1993;90:1513–1517. doi: 10.1073/pnas.90.4.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikada Y, Tabata Y. Protein release from gelatin matrices. Adv Drug Deliv Rev. 1998;31:287–301. doi: 10.1016/s0169-409x(97)00125-7. [DOI] [PubMed] [Google Scholar]
- 32.Tabata Y, Hijikata S, Muniruzzaman M, et al. Neovascularization effect of biodegradable gelatin microspheres incorporating basic fibroblast growth factor. J Biomater Sci Polym Ed. 1999;10:79–94. doi: 10.1163/156856299x00298. [DOI] [PubMed] [Google Scholar]
- 33.Tabata Y, Miyao M, Yamamoto M, et al. Vascularization into a porous sponge by sustained release of basic fibroblast growth factor. J Biomater Sci Polym Ed. 1999;10:957–968. doi: 10.1163/156856299x00559. [DOI] [PubMed] [Google Scholar]
- 34.Marui A, Tabata Y, Kojima S, et al. A novel approach to therapeutic angiogenesis for patients with critical limb ischemia by sustained release of basic fibroblast growth factor using biodegradable gelatin hydrogel: an initial report of the phase I-IIa study. Circ J. 2007;71:1181–1186. doi: 10.1253/circj.71.1181. [DOI] [PubMed] [Google Scholar]
- 35.Miyoshi M, Kawazoe T, Igawa HH, et al. Effects of bFGF incorporated into a gelatin sheet on wound healing. J Biomater Sci Polym Ed. 2005;16:893–907. doi: 10.1163/1568562054255709. [DOI] [PubMed] [Google Scholar]
- 36.Uchi H, Igarashi A, Urabe K, et al. Clinical efficacy of basic fibroblast growth factor (bFGF) for diabetic ulcer. Eur J Dermatol. 2009;19:461–468. doi: 10.1684/ejd.2009.0750. [DOI] [PubMed] [Google Scholar]
- 37.Kanda N, Morimoto N, Takemoto S, et al. Efficacy of novel collagen/gelatin scaffold with sustained release of basic fibroblast growth factor for dermis-like tissue regeneration. Ann Plast Surg. 2012;69:569–574. doi: 10.1097/SAP.0b013e318222832f. [DOI] [PubMed] [Google Scholar]


