Abstract
Background:
As the elderly population continues to expand, it becomes increasingly important to develop treatments to improve wound healing in the elderly. One problem limiting the research is the lack of appropriate animal models for wound healing in elderly patients. We hypothesized that the Klotho mouse of premature aging is a suitable animal model to shed light on many of the biological processes involved in aging skin.
Methods:
Klotho mice (kl/kl), Klotho-heterozygous mice (kl/+), and wild-type mice (+/+) were wounded, and the area of the wound was measured every 3 days until the wound was healed. To compare the klotho phenotype with wild-type mice, wounds were also harvested at 4 and 7 days after wounding. For histological examination, paraffin-embedded sections were stained with hematoxylin and eosin and Masson trichrome. Collagen expression in the wound was also studied by analyzing messenger RNA using real-time polymerase chain reaction.
Results:
Klotho mice showed a significantly slower rate of wound closure compared with Klotho-heterozygous mice and wild-type mice. Histology showed substantial less healing and collagen deposition in the wounds of the Klotho mice. The expression of collagen messenger RNA in Klotho mice was also less than that in heterozygous and wild-type mice. The Klotho mice exhibited significant phenotypic similarities with aged skin, such as atrophy and delayed wound healing.
Conclusion:
These preliminary data suggest that the Klotho mouse may be a model to further investigate wound healing in the elderly.
Compared with normal healthy adults, elderly patients have many difficulties with wound healing. Their nonhealing ulcers and wounds often cause serious infections that can be fatal. As there are very few appropriate animal models of the human aging process, very little is known about the molecular mechanisms of human aged skin and wound healing.1 However, a novel mouse model that exhibits multiple aging phenotypes has been developed in the past 20 years by disrupting the Klotho gene.2
A defect in Klotho gene expression in mice results in a syndrome that resembles premature aging. The mice develop normally during the first 3 weeks. Thereafter, they gradually become inactive and die prematurely at 8–9 weeks. The Klotho mutant mouse (kl/kl), which carries a deletion mutant allele disrupted by an insertional transgene, is the first laboratory animal model for human aging caused by a single gene mutation.3 A defect in Klotho gene expression in the mouse exhibits a progeric phenotype, including shortened life span, infertility, emphysema, atherosclerosis, osteoporosis, and skin atrophy.2 Despite extensive experimental studies to date, information available for understanding the pathophysiological role of the Klotho gene is still limited, and much more remains to be clarified.
We hypothesized that the Klotho mouse is a good animal model to illuminate many of the biological processes involved in wound healing of aging skin. This series of experiments aims to (1) compare the phenotype of the Klotho mice with wild-type counterparts and identify similarities between Klotho mice and aged skin and (2) determine the rate of wound healing in the Klotho mice.
METHODS
Animals
Genetically Klotho-heterozygous mice (kl/+) were obtained from Clea-japan (Tokyo, Japan).
Animals were housed in the University of California, San Francisco Animal Care Facility. The Committee on Animal Research approved all procedures. All mice were between 4 and 5 weeks of age when wounded.
Genotyping Animals
Each genotype of newborn Klotho mice (kl/kl) from the mating male and female heterozygous was obtained through reproduction by many pairs of heterozygous parents (Fig. 1). Genotyping was done using the polymerase chain reaction (PCR) procedure with the primers: 5′-TGGAGATTGGAAGTGGACG-3′, 5′-CAAGGACCAGTTCATCATCG-3′, and 5′-TTAAGGACTCCTGCATCTGC-3′, which discriminate the homozygotes of Klotho mice from other genotypes. The DNA separated from the tail of each mouse was amplified with Taq DNA polymerase (Qiagen). Amplification cycles ran 30 times, with each cycle composed of 30 seconds at 94°C, 30 seconds at 56°C, and 90 seconds at 72°C. Mutated and wild alleles were visualized as signals at 920 and 458 bp, respectively, with heterozygous genotypes showing both signals.
Figure 1.

Upper, wild-type mouse; lower, Klotho mutant mouse. The Klotho mouse is smaller in size and has kyphosis.
Wounding and Measurement of Wound Closure
We studied wild-type (+/+), Klotho-heterozygous (kl/+), and Klotho (kl/kl) mice. Each mouse was given its own cage, a day before the experiment. Five animals in each group were anesthetized with inhalation isoflurane. We used a heat pack (All Temp Therapy) to keep the animals from getting hypothermia. The dorsum of the mouse was shaved and sterilized with an alcohol pad, and a single 0.8-cm full-thickness open wound was excised including the panniculus carnosus layer (Fig. 2). The open wounds were measured immediately after the wounding, measured again the following day, and then every 3 days until the wound was healed. Planimeter was used to determine the area of the wound tracing. The areas of the wounds were quantitatively analyzed using Adobe Photoshop CS software (Adobe, San Francisco, Calif.). For wound biopsy purposes, animals received a single 0.8-cm wound. Animals were euthanized either 4 or 7 days after the wounding, at which time the wounds were harvested and processed for isolating RNA and histological examination. A total of 2 wild-type mice, 2 kl/+ mice, and 2 kl/kl mice were used at each time point.
Figure 2.
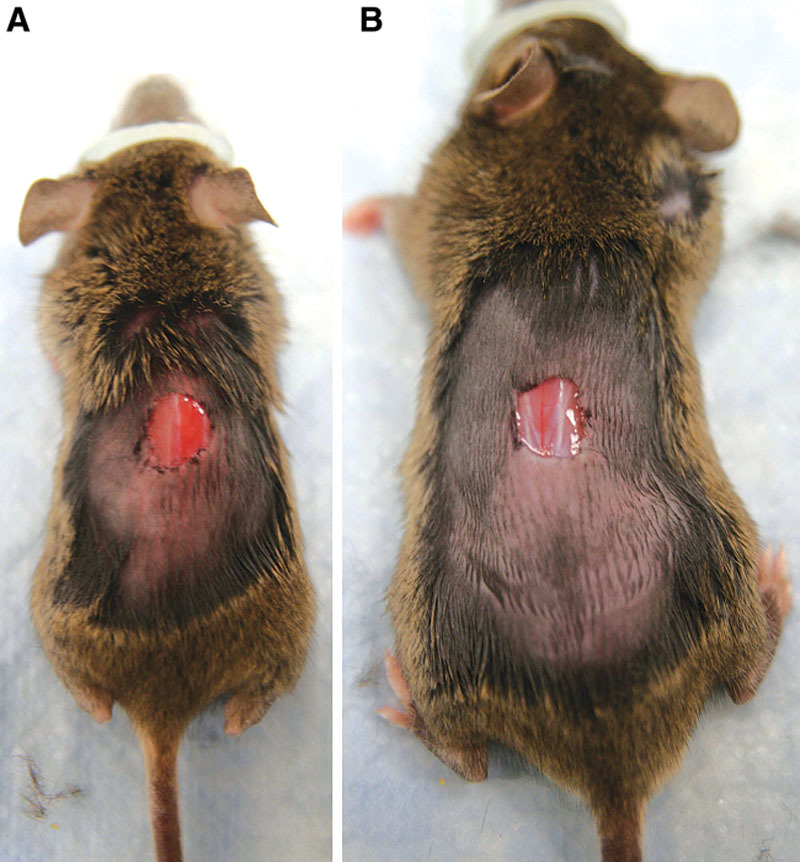
Wounding experiment. A, Klotho mouse. B, Wild-type mouse. Each animal has 0.8-cm open wound on the dorsum.
Histological Examination
Excised skins were fixed with 10% formaldehyde overnight at room temperature. Tissues were then dehydrated in ethanol, embedded in paraffin, and sectioned into 5-μm slices. Slides were then stained with hematoxylin and eosin (H&E) or Masson-Goldner trichrome staining.
Histological Score
Wounds (0.8 cm) were harvested 4 and 7 days after wounding. Histological scores were assigned in a blinded manner according to the method of Greenhalgh et al.4 Briefly, each specimen was given a score of 1–12: 1–3, none to minimal cell accumulation and granulation tissue or epithelial migration; 4–6, thin, immature granulation dominated by inflammatory cells but with few fibroblasts, capillaries, or collagen deposition and minimal epithelial migration; 7–9, moderately thick granulation tissue, ranging from being dominated by inflammatory cells to more fibroblasts and collagen deposition; and 10–12, thick, vascular granulation tissue dominated by fibroblasts and extensive collagen deposition.
RNA Isolation and Real-time PCR
Mouse wound tissues were snap frozen at –80°C then subsequently homogenized before RNA isolation using TRIZOL Reagent (Life Technologies, Rockville, MD). Complementary DNA was synthesized from 500 ng of total RNA using a TaqMan Gold RT-PCR Kit (Applied Biosystems) according to the manufacturer’s protocol. The complementary DNA samples were diluted 20-fold, and real-time PCR reaction was carried out with 100 μM of primer. Amplifications were performed in an ABI PRISM 7000 Sequence Detection System (Applied Biosystems). The thermal cycler conditions were 50°C for 2 minutes and 95°C for 10 minutes to activate or inactivate various enzymes, and then 40 cycles each of 15 seconds at 95°C (denaturation) followed by 1 minute at 59°C (annealing and extension). The 18S plasmid was used as standard DNA. All standards and samples were assayed in triplicate. The threshold cycle values were used to plot a standard curve. All samples were normalized to the relative levels of 18S, and results were expressed as fold-increase. Microsoft Excel was used for data analysis.
Statistical Analysis
The values presented are mean + SD unless otherwise specified. Statistical significance was evaluated by paired Student’s t test when appropriate. A probability value < 0.05 was considered significant.
RESULTS
Klotho Mice Show Impaired Wound Healing
To determine whether Klotho mice (kl/kl) would be the appropriate wound-healing model for aged skin, we made a 0.8-cm open wound and measured for closure. Representative photographs of the wounds in Klotho mice 19 days after wounding continued to show larger wounds than those of control wild-type mice (Fig. 3).
Figure 3.
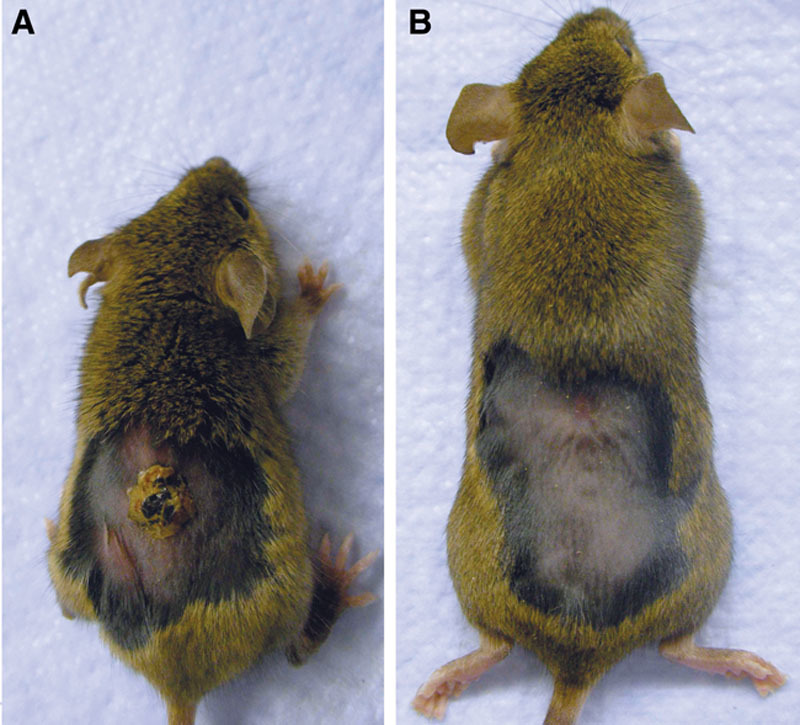
Nineteen days post wounding.
We found that the wounds on the kl/kl mice were statistically significantly larger wounds from day 1 until closure than those on wild-type mice (Fig. 4). On average, the control wild-type mice and the kl/+ mice wounds closed by day 19 (±1 days), whereas kl/kl mice wounds required 2 more weeks, at day 35 to show complete closure. Interestingly, the kl/kl mice wound did not show any contraction; although it was enlarged in first 5 days, then it closed slowly up to day 35.
Figure 4.
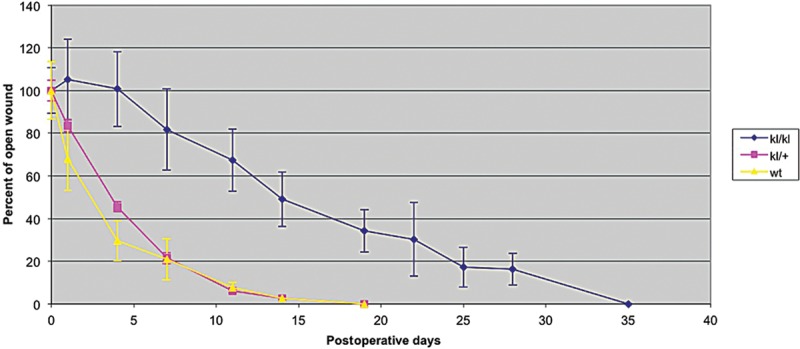
Wound closing curve. Wt, wild-type mice.
However, Klotho mice often died unexpectedly under a variety of stressful conditions, such as transportation, anesthesia, and high physical activity. We made 5 more wounds in each of the Klotho mice for the wounding experiment, but most died within a week due to stressful conditions. The mortality rate of Klotho mice would be 50% compared with 0% on wild-type and heterozygous mice.
Histological Difference of Klotho Mice and Wild-type Mice Skin
To confirm substantial difference in skin between Klotho mice and wild-type mice, H&E staining was performed on skin biopsies harvested from the animals (Fig. 5). Compared with the normal wild-type mice skin, the Klotho mice showed decreased numbers of hair follicles, reduction of dermal and epidermal thickness, and no appearance of subcutaneous fat, which is very similar to the feature of senile atrophoderma in humans.
Figure 5.
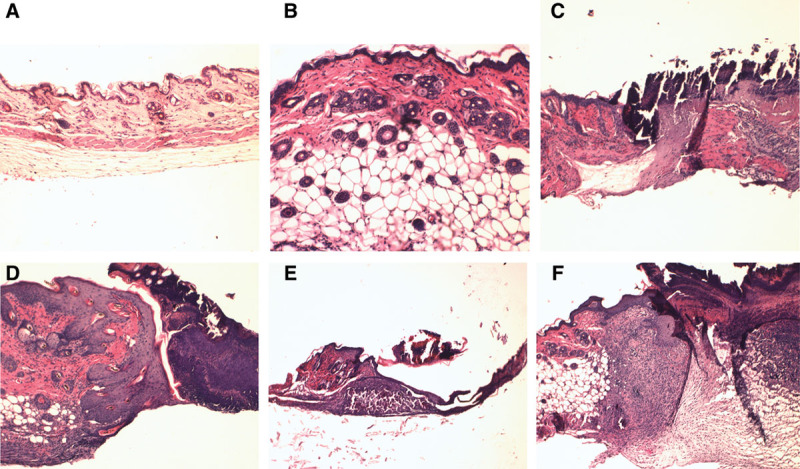
A, Klotho mouse skin. B, Wild-type skin. C, Klotho mouse skin wound day 4. D, Wild-type mouse skin wound day 4. E, Klotho mouse skin wound day 7. F, Wild-type mouse skin wound day 7.
Wound Appearance at 4 and 7 Days after Wounding
On day 4 and day 7 after wounding, we can see the differences in the appearance of wounds. We stained wound biopsies harvested from animals 4 and 7 days after wounding with H&E staining and Masson trichrome staining (Fig. 5). Klotho wounds displayed a decreased amount of granulation tissue, collagen deposition, and inflammatory cells when compared with the wild-type wounds at both days 4 and 7. The impairment in wound appearance and granulation tissue was quantified by blind assignment of a histological score to wound sections at 4 and 7 days following wounding (Fig. 6).
Figure 6.
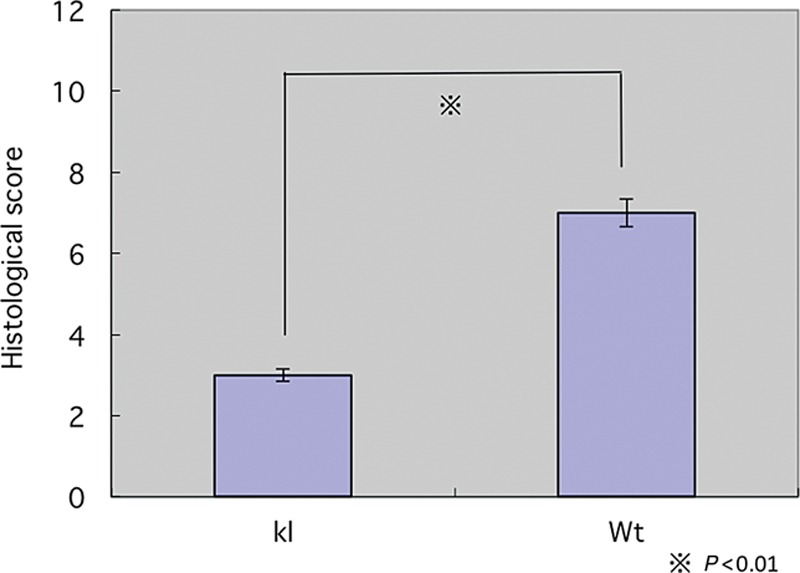
Histological score. Wt, wild-type mice; kl, Klotho mice.
Collagen Expression on Klotho and Wild-type Wound
The collagen content of the dermis and the rate of collagen production are both decreased with aging. A deficit of type 1 and type 3 collagen in wound tissue occurs. To answer the question of whether a Klotho mice wound has the same characteristics as aged skin wounds, we isolated messenger RNA from both days 4 and 7 wound tissue and analyzed the material with semiquantitative real-time PCR. As shown in Figure 4, type 1 and 3 collagen messenger RNA is decreased in expression in Klotho mice compared with both heterozygous and wild-type mice (Fig. 7). We also confirmed this conclusion histologically (Fig. 8). Masson trichrome staining showed that collagen deposition was reduced in Klotho mice when compared with wild-type mice (Fig. 8).
Figure 7.
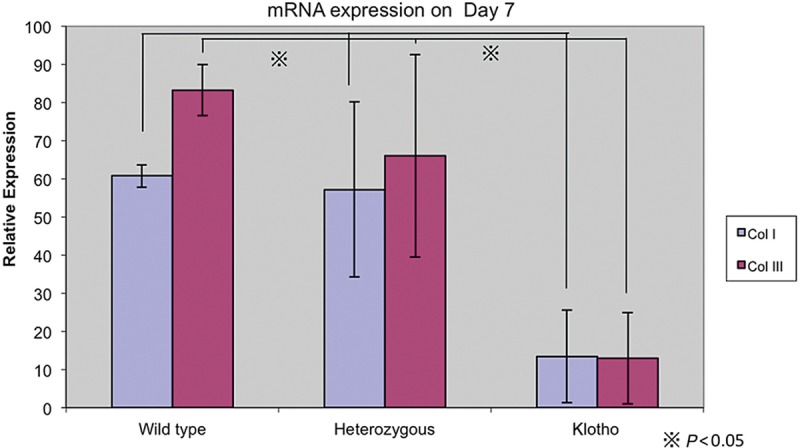
Collagen messenger RNA (mRNA) expression on day 7 wound.
Figure 8.
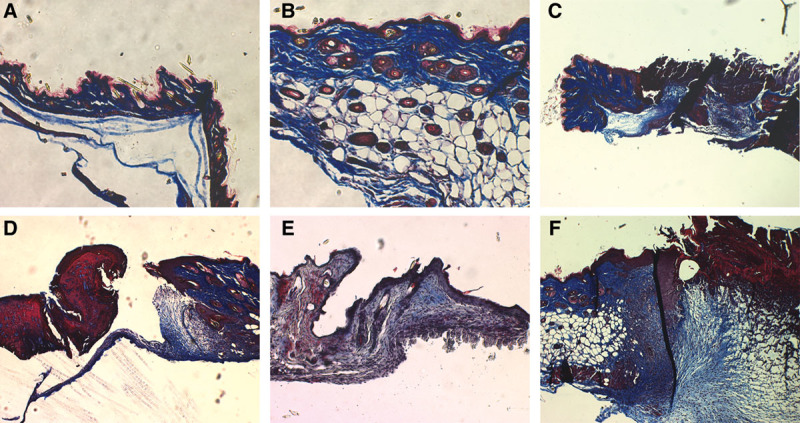
A, Klotho mouse skin. B, Wild-type mouse skin. C, Klotho mouse skin wound day 4. D, Wild-type skin wound day 4. E, Klotho mouse skin wound day 7. F, Wild-type skin wound day 7.
DISCUSSION
Although novel mouse models that exhibit multiple aging phenotypes have been developed in the past few years, very few manifest the phenotype of aged skin characterized by atrophy, drying, roughness, alterations in pigmentation, sagging, and wrinkling.1,5,6 Other mouse models, except aged animals, exhibit limited characteristics important to aged skin. Aged mouse models showing skin changes are senescence-accelerated mice and telomerase-deficient mice. Hypophysectomized mice exhibit reduced replicative potential of fibroblasts in vitro. Their genetics seem to be very complex, and the phenotypes are also limited. Studying aged animals in wound-healing models presents logistical difficulties.
We are interested in Klotho mice as the animal model for wound healing of aged skin. The klotho gene is known to prolong life and prevent onset of many aged phenotypes.2,7 When this gene is disrupted as in the Klotho mutant mouse (kl/kl), animals exhibit a progeric phenotype which includes shortened life span, infertility, emphysema, atherosclerosis, osteoporosis, and skin atrophy.2,8–10 Klotho mice not only exhibit the phenotypes that reflect the aspects of aging but also sustain age-related disorders commonly observed in humans, such as arteriosclerosis and osteoporosis.7,11 The Klotho mouse is regarded as the first laboratory animal model for human aging in general that is caused by a single gene mutation.
The results of this study show that Klotho mice exhibit significant phenotypic similarities with aged skin consistent with delayed wound healing and skin atrophy. Using the excisional open wound model, we have observed that Klotho mice display substantially delayed healing, similar to normally aged mice. Compared with wild-type mice, Klotho mice require twice as long as to show complete closure. Klotho mice were found to exhibit some degree of delay in re-epithelization, collagen synthesis, and angiogenesis. These data are in agreement with previous reports that describe an age-related delay in wound healing.5,6,12,13
In addition to delays in healing, our study showed an obvious delay in collagen deposition in wounds from Klotho mice. The previous report stated that despite no significant effect of age on collagen content of wounds, there was an apparent lag in collagen production during the proliferate phase in aged mice.6,14 Our data also show that there is significant difference in expression of collagen genes, paralleling this delay on collagen deposition. This suggests that the Klotho mouse is a suitable model to further investigate wound healing in the elderly.
Unfortunately, the premature aging of the Klotho mice makes the animal more vulnerable to death and presents some obstacles to the study. As Klotho mice are very vulnerable to stresses such as transportation, anesthesia, or wounding, they are fragile for skin wounding experiments.15 We also experienced a high mortality rate at over 50%, which presented a further hindrance to the study. However, with conservative care during anesthesia and surgery as described below, it is possible to obtain a lower mortality rate and a better wound-healing model for the study of aged skin. We recommend the following.
Avoiding hypothermia: We always place heat packs (All Temp Therapy) underneath the mouse during anesthesia and surgery. These packs keep the temperature at nearly 54°C for about half an hour so that the mice can keep warm for a couple hours after they go back in the cage.
Working quickly: To shorten the time of physical stress such as anesthesia and surgery, it is very important to prepare all needed materials before you start an experiment on Klotho mice. This can significantly decrease the mortality rate.
Using appropriate amount of anesthesia drugs: As Klotho mice are smaller than same aged wild-type mice, an overdose of anesthetic can accidentally be administered while the mouse is undergoing surgery. Generally, 2–3% isoflurane in oxygen at 2 L/min was adequate for wild-type mice, while a reduced amount of 1–1.5% gas would be suitable for Klotho mice.
Not doing the experiment on subjects over the age of 6 weeks: Klotho mice begin to manifest multiple age-related disorders from the age of 4 weeks.13 As they suffer premature death around 2 months of age, an age difference of a week causes a higher mortality rate. Five weeks of age is the most proper time to wound the mice.
Soaking the food: It gets hard for Klotho mice to gnaw and chew after they have started to display the aging phenotypes. Dipping food pellets in the water helps them to eat the appropriate amount of food.
Separating the subjects to individual cages a day before experiment: Transportation can be very stressful for Klotho mice. Both wounding and separating them on same day would cause a higher mortality rate, so to lower this possibility, the mice should be separated a day before the procedure.
Preparing for nesting: It is also important to provide them with an adequate amount of material for nesting. This prevents hypothermia and is useful for health checks of the mice.
Particularly, avoiding hypothermia and keeping the mice warm during and after wounding are important. Because of the body size of Klotho mice, even with a smaller wound, they can easily get hypothermia, which leads to premature death. It is necessary to keep a suitable body temperature with minimum influence to the experimental site.
Although the Klotho mouse is suitable for the study of delayed healing in acute wounds in the elderly, it is difficult to study about chronic wounds because the mice die in a short period. Morishita16 reported that changing their nutrition to dietary phosphorus and zinc resulted in lengthening of their lifespan, but there is a concern that the change in nutrition might affect the wound-healing process. It might not represent a chronic wound, and experiments would be difficult.
To put it simply, we fully realize a need for much more intensive care for the Klotho mice, much like an elderly person, than the wild-type mice in the experimentation.
Although it needs such care, there is no aged wounding model showing all of the phenotypes of aged skin characterized by atrophy, drying, roughness, alterations in pigmentation, sagging, and wrinkling. Moreover, such care is similar to that for an elderly person, as it takes much time but is not complicated. By following these recommendations, we could use the Klotho mouse as an aged mouse wound model. This is an important step for aging research because the Klotho mouse shares common molecular mechanisms with aged people and can thereby broaden our knowledge of wound healing in the elderly.
CONCLUSION
Our data suggest that the Klotho mouse may be a model to further investigate wound healing in the elderly.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Kuro-o M. Disease model: human aging. Trends Mol Med. 2001;7:179–181. doi: 10.1016/s1471-4914(01)01921-9. [DOI] [PubMed] [Google Scholar]
- 2.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 3.Kuroo M. Introduction: aging research comes of age. Cell Mol Life Sci. 2000;57:695–697. doi: 10.1007/s000180050033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenhalgh DG, Sprugel KH, Murray MJ, et al. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol. 1990;136:1235–1246. [PMC free article] [PubMed] [Google Scholar]
- 5.Davidson JM. Animal models for wound repair. Arch Dermatol Res. 1998;290(Suppl):S1–11. doi: 10.1007/pl00007448. [DOI] [PubMed] [Google Scholar]
- 6.Gosain A, DiPietro LA. Aging and wound healing. World J Surg. 2004;28:321–326. doi: 10.1007/s00268-003-7397-6. [DOI] [PubMed] [Google Scholar]
- 7.Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manya H, Akasaka-Manya K, Endo T. Klotho protein deficiency and aging. Geriatr Gerontol Int. 2010;10(Suppl 1):S80–S87. doi: 10.1111/j.1447-0594.2010.00596.x. [DOI] [PubMed] [Google Scholar]
- 9.Liu H, Fergusson MM, Castilho RM, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 10.Lanske B, Razzaque MS. Premature aging in klotho mutant mice: cause or consequence? Ageing Res Rev. 2007;6:73–79. doi: 10.1016/j.arr.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nabeshima Y. Toward better understanding of klotho. Sci Aging Knowl Environ. 2006;8:pe11. doi: 10.1126/sageke.2006.8.pe11. [DOI] [PubMed] [Google Scholar]
- 12.Brem H, Tomic-Canic M, Entero H, et al. The synergism of age and db/db genotype impairs wound healing. Exp Gerontol. 2007;42:523–531. doi: 10.1016/j.exger.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Yanai H, Budovsky A, Tacutu R, et al. Is rate of skin wound healing associated with aging or longevity phenotype? Biogerontology. 2011;12:591–597. doi: 10.1007/s10522-011-9343-6. [DOI] [PubMed] [Google Scholar]
- 14.Swift ME, Kleinman HK, DiPietro LA. Impaired wound repair and delayed angiogenesis in aged mice. Lab Invest. 1999;79:1479–1487. [PubMed] [Google Scholar]
- 15.Takeshita K, Fujimori T, Kurotaki Y, et al. Sinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expression. Circulation. 2004;109:1776–1782. doi: 10.1161/01.CIR.0000124224.48962.32. [DOI] [PubMed] [Google Scholar]
- 16.Morishita K, Shirai A, Kubota M, et al. The progression of aging in Klotho mutant mice can be modified by dietary phosphorus zinc. J Nutr. 2001;131:3182–3188. doi: 10.1093/jn/131.12.3182. [DOI] [PubMed] [Google Scholar]


