Summary
Objective
A significant risk factor for anterior cruciate ligament (ACL) tears in young athletes is a reduced femoral Notch Width Index (NWI). The purpose of this study was to test if persons with knee osteoarthritis (OA) and ACL tears have smaller NWI independent of prior joint injury and osteophyte volume.
Methods
We included 160 participants from the progression sub-cohort of the Osteoarthritis Initiative (OAI) Study, an ongoing 4-year, multi-center study, focusing on knee OA. The femoral notch width, the condylar notch width at 2/3 of the notch depth, and the intercondylar notch angle (β) were measured on sagittal and coronal MR-images. NWI = notch width/condylar width at 2/3 of the notch depth, was calculated and outcome of ACL tear frequency was compared between two groups; NWI ≤ 0.20 and NWI > 0.20. The NWI and β were analyzed as continuous variables.
Results
Of the 160 subjects [51% female, age 62.1 (±9.9), BMI 30.3 (±4.7) kg/m2] 14.4% showed an ACL tear. Osteophyte bone volume was available for 150 participants, of which 13% had an ACL tear. The continuous measure of NWI on the coronal images was significantly (P = 0.01) smaller in participants with ACL tear [0.246, 95% confidence interval (CI) 0.234–0.258] compared to those without (0.263, 95% CI 0.258–0.268). Adjustment for demographic variables still showed significant results (P = 0.03, mean difference 0.015 95% CI −0.001–0.030) and adjustment for demographic variables and osteophyte bone volume were borderline significant (P = 0.06, mean difference 0.015 95% CI 0.001–0.029).
Conclusions
We identified a smaller NWI in participants with knee OA and ACL tears. Further longitudinal investigation is necessary to determine this as an independent risk factor.
Keywords: Knee OA, ACL tear, Notch stenosis, NWI
Introduction
Osteoarthritis (OA) is a significant public health challenge with a large burden for health care services1 and the leading cause of disability in elders2. OA affects up to 30% of the western population over the age of 653. Symptomatic OA of the knee occurs in 6% of adults 30 years of age or older2, and in 13% of persons age 60 and over4.
Among those with symptomatic knee OA, between 22 and 35%5–7 have incidental complete anterior cruciate ligament (ACL) tears identified by magnetic resonance imaging (MRI). The majority of persons with this finding have no recollection of prior trauma or substantive knee injury likely to predispose to ACL tear7. Rupture of the ACL is known to lead to premature OA in young, athletic participants8–12, due to subsequent anteroposterior instability of the knee and can accelerate the rate of OA progression13.
One purported confounder for spontaneous rupture in young athletes is a reduced width of the femoral intercondylar notch14,15; measured as a smaller Notch Width Index (NWI)16 on radiographs and CT scans. Smaller notch width, notch angles and notch surfaces have also been found in ACL deficient OA knees17. Furthermore, osteophyte growth in the notch area appears to correlate with progression of medial tibiofemoral knee OA18. Leon et al.19 suggested that intercondylar notch stenosis may be a proximate cause of ACL damage, symptomatic instability and loss of extension in participants with knee OA. These prior studies however all used convenience samples who presented to surgical centers for operative intervention. Furthermore it has been demonstrated, that in knees with acute ACL tears chondral lesions seem to appear more frequently in the lateral tibiofemoral compartment20 and that contact pattern of ACL injured knees compared to healthy controls was shown to be more posterior in acutely injured knees. This also seemed to be associated with severity of symptoms21,22. Therefore ACL tears in persons with knee OA might be associated with a different pattern of disease progression, including increased cartilage loss, meniscal degeneration or specific patterns of bone marrow lesion location. Thus knowing about a smaller notch width and therefore predisposition for an ACL tear that is associated with a specific pattern of disease, might lead to recommendations about lifestyle counseling, prophylactic knee bracing and sport restrictions. At present little is known about the relation between the NWI, the notch angle and the prevalence of ACL tears in OA participants, unselected for ACL integrity. Given the impact of ACL tears on OA progression it is important to identify potential proximate confounders or risk factors.
Identification of notch stenosis as an associated confounder for ACL tears in persons with knee OA, would be helpful in delineating this phenotype as one at risk for ACL tear and possibly the antecedent confounder for OA progression or a different pattern of disease.
With this rationale in mind, the objective of this study was to examine cross-sectionally whether femoral notch stenosis is associated with ACL tears in persons with knee OA. Our first hypothesis was that ACL tears will be associated with a higher frequency of preexisting femoral notch stenosis. Furthermore our second hypothesis was that persons with prevalent ACL tear will have a smaller NWI independent of demographic factors and volume of notch osteophytes and joint injury.
Materials and methods
STUDY SAMPLE
Subjects included in this analysis are a subset of the 4796 participants participating in the Osteoarthritis Initiative (OAI) Study, which is an ongoing multi-center longitudinal observational study, focusing primarily on knee OA. The study protocol, amendments, and informed consent documentation were reviewed and approved by the local institutional review boards. Data used in the preparation of this manuscript were obtained from the OAI database, which is available for public access at http://www.oai.ucsf.edu/. The specific datasets used are clinical dataset 0.1.1 and Image Release 0.B.1.
OAI consists of two sub-cohorts: the Progression sub-cohort and an incidence sub-cohort. 389 participants with radiographic signs and symptoms of knee OA at baseline were recruited into the Progression sub-cohort and another group at risk for symptomatic knee OA was recruited to the Incidence sub-cohort. All of the participants for the present study are drawn from the Progression sub-cohort.
The inclusion criteria for the Progression sub-cohort of the OAI required that both of the following criteria must be present together in at least one knee at baseline:
Frequent knee symptoms, defined as pain, aching or stiffness on most days of the month during the past year and
Radiographic Osteoarthritis (ROA) defined as definite tibiofemoral osteophytes (Osteoarthritis Research Society International (OARSI) atlas grade ≥1). Subjects with severe tibiofemoral (joint space narrowing (JSN) OARSI grade 3 narrowing or bone on bone) in both knees were excluded. The grading of osteophytes and JSN was done at each individual OAI enrollment center.
Due to the available data from a prior study23 that included ACL readings done by a board certified musculoskeletal radiologist, and the convenience of the study size, 160 subjects from the OAI Progression sub-cohort were included in this analysis. These were selected by OAI from participants who had complete baseline and 1 year MRI data in early 2006, with blocking for sex and center for this prior study23.
The demographic characteristics of the study sample are shown in Table I. Fifty-one percent of the study population were female, the average age of the participants was 62.1 years with a standard deviation (SD) of 9.9 years, 59% of the participants were 65 years of age or older. The mean body mass index (BMI) was 30.3 kg/m2 with a SD of 4.7. The left knee was chosen as the index knee in 78 (49%) participants. If graded by the Kellgren & Lawrence grade (KLG) 15% of the participants observed in this study sample did not have radiographic OA (ROA) defined by a KLG ≥ 2. This fact is made possible by the eligibility criteria used for the OAI Progression sub-cohort which was based on the identification of a definite tibiofemoral osteophyte by the individual OAI enrollment center and some disagreement in radiographic assessment with the adjudicated central reading scoring was expected.
Table I.
Descriptive characteristics of study sample (n = 160)
| All | No ACL (137) | ACL (23) | |
|---|---|---|---|
| Gender [female N (%)] | 81 (51) | 76 (56) | 5 (22) |
| Age (mean, SD), years | 62.1 (9.9) | 62.9 (9.5) | 57.5 (10.8) |
| Age group [≤65 years N (%)] | 95 (59) | 77 (56) | 18 (78) |
| BMI (mean, SD), kg/m2 | 30.3 (4.7) | 30.3 (4.7) | 30.5 (4.8) |
| Interval between visit [mean (SD), median, range-days] | 387.3 (49.7), 386, 76–546 | 388 (43.0), 384.5, 86–546 | 380 (81.7), 387, 76–489 |
| Index knee [left N (%)] | 78 (49) | 69 (50) | 9 (39) |
| KLG of index knee, no. (%) | |||
| 0 | 6 (4) | 6 (4) | 0 (0) |
| 1 | 18 (11) | 17 (12) | 1 (4) |
| 2 | 58 (36) | 51 (37) | 7 (30) |
| 3 | 64 (40) | 54 (39) | 10 (44) |
| 4 | 14 (9) | 9 (7) | 5 (22) |
| History of knee and hip surgery including arthroscopy [yes for study knee N (%)] | 45 (28.9) | 31 (22.6) | 14 (60.9) |
| History of knee injury [yes for study knee N (%)] ever injured badly enough to limit ability to walk for at least 2 days | 75 (47.2) | 53 (38.7) | 22 (95.7) |
A partial or complete ACL tear was present in 23 study participants with 22 of these participants reporting a history of substantive knee injury.
ASSESSMENT OF JOINT INJURY AND SURGERY
During the enrollment visit, history of joint surgery was evaluated by asking the participants if they ever had any kind of knee surgery including arthroscopy, ligament repair surgery or meniscectomy, or if they ever had a hip replacement surgery. Furthermore participants have been asked separately for both sides, if they ever injured their knee badly enough to limit their ability to walk for at least 2 days. Results from this survey for our study population can be found in Table I.
RADIOGRAPHIC ASSESSMENT
Bilateral fixed-flexion posteroanterior (PA) views of the knee were obtained, using a SynaFlexer™ frame (Synarc, Inc., San Francisco, CA)24.
Baseline radiographs of the 160 subjects were read independently by two readers, one a bone and joint radiologist (AG), and the other a rheumatologist (DH)23. Readers evaluated the KLG on a 0–4 scale25. The KLG adjudicated readings were arrived at by a consensus of the readers. Disagreements on JSN were also adjudicated if the two readers disagreed.
SELECTION OF KNEE FOR ANALYSIS
One knee from each of the 160 participants provided by OAI was included for analysis. The selection of the index knee for this analysis was based on the presence of both symptoms (frequent knee pain) and radiographic OA (ROA) in the same knee and likelihood of progression as previously described23.
MRI SEQUENCE PARAMETERS
For this study only the baseline exams were used. All Images were acquired on a 3 T MRI scanner (Siemens Magnetom Trio, Erlangen, Germany) with a quadrature transmit-receive knee coil (USA Instruments, Aurora, OH). For the purposes of the notch measurements we used the sagittal 3D dual echo in the steady state (DESS) with water excitation (SAG_3D_DESS) images with a slice thickness of 0.7 mm, 16.3 ms turnover rate (TR), 4.7 ms echo time (TE), 25° flip angle, 14 cm × 14 cm field of view (FOV); 384 × 307 pixels matrix; 185 Hz/pixel bandwidth, 1 average, anterior/posterior phase encoding, (acquisition time 10 min 23 s) and the axial multiplanar-reformatted (MPR) images of the 3D DESS sequence.
For the coronal images we used the coronal intermediated weighted turbo spin echo (IW TSE) (COR_IW_TSE) with a slice thickness of 3 mm, 3850 ms TR, 29 ms TE, 180° flip angle (FA), 14 cm × 14 cm FOV; 384 × 307 pixels matrix; 352 Hz/pixel bandwidth; acquisition time 3 min 24 s.
MRI READINGS
ACL tears
MRIs were read for ACL tears, by one board certified musculoskeletal radiologist (AG)23. The presence of an ACL tear at baseline was read using sagittal and coronal views and scored on a 0–2 scale, with 0 = normal, 1 = partial tear and 2 = complete tear. A complete tear was defined as complete disruption of ACL fibers with ligament discontinuity, while a partial tear was defined as a residual straight and tight ACL fiber in at least one pulse sequence. The weighted kappa for intra-observer reliability was 0.75. For the purposes of this analysis all tears, whether partial or complete, were considered torn.
Femoral notch stenosis
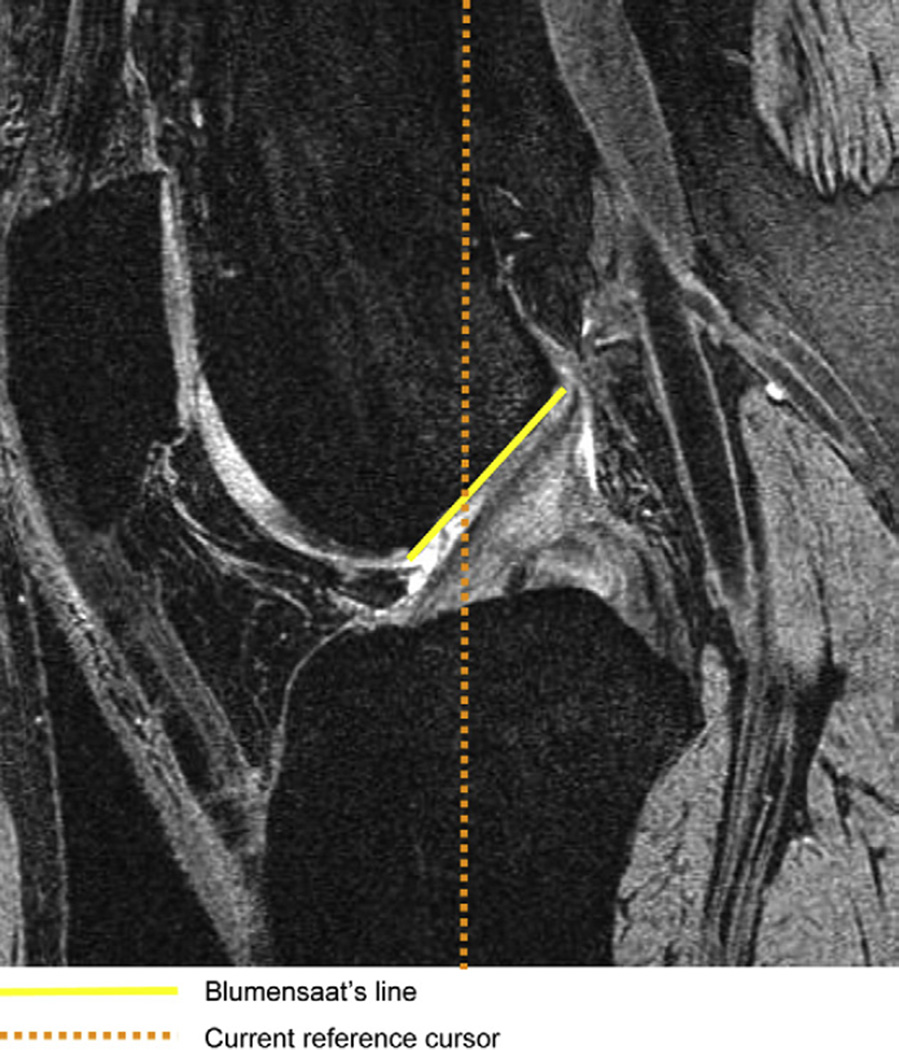
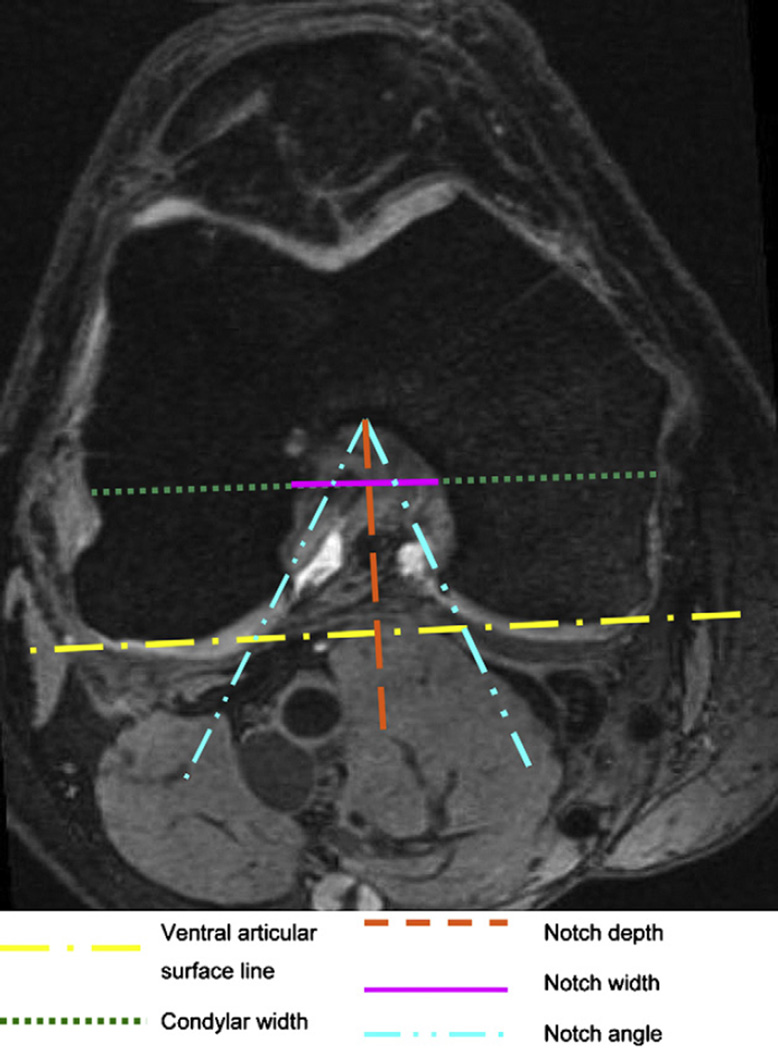
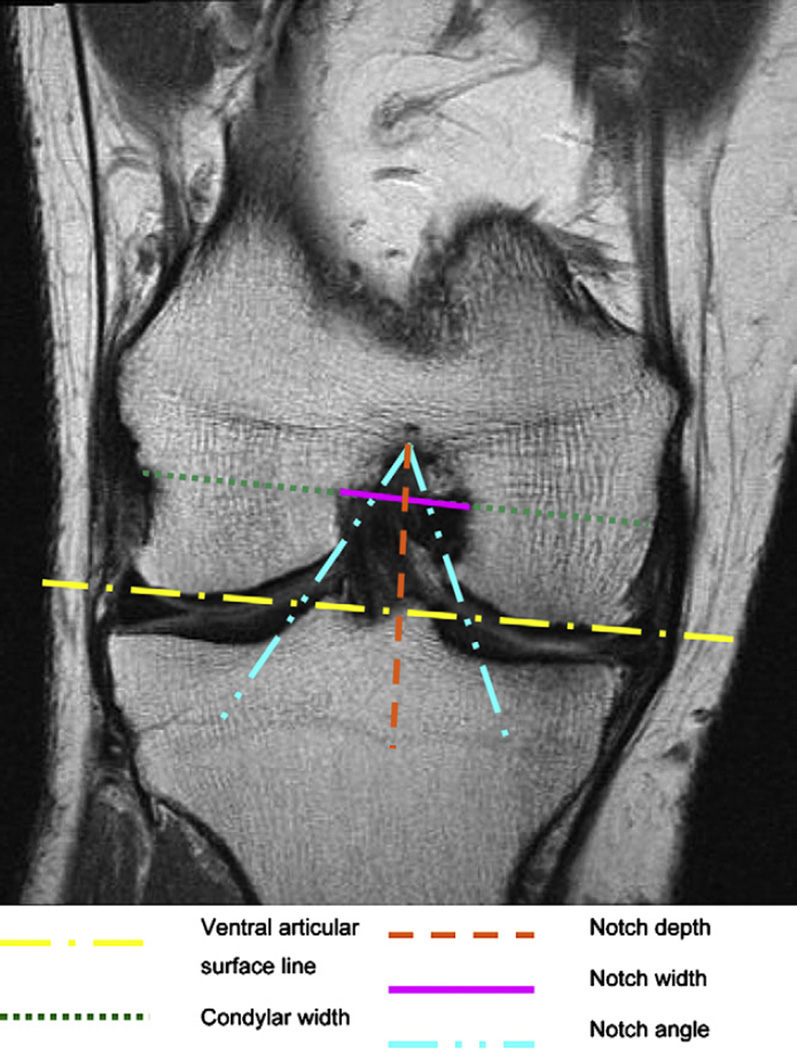
The appropriate level on the axial and coronal sequences was defined, by using the sagittal sequence. For this purpose we marked the anterior outlet of the intercondylar notch on the sagittal image by drawing the Blumensaat’s line (intercondylar roof line) and moved the “current reference cursor” to the beginning of the Blumensaat’s line, as shown in Fig. 1. For the purpose of this study we measured the femoral notch width and condylar width at 2/3 of the notch depth, on a line perpendicular to the notch depth, and parallel to a line connecting the ventral articular surfaces of the medial and the lateral femoral condyle on both the axial and coronal images, as shown in Figs. 2 and 3. Furthermore we measured the notch angle. As depicted in the literature14,26 the critical value for the notch angle was 50°. All measurements were made in mm or degrees, using Merge Efilm software. The NWI = notch width/condylar width, was calculated separately for the coronal and axial sequences using the notch width and condylar width as described above. Participants were divided in two groups, one with notch stenosis, one without, using 0.20 as the critical value27. All measurements where made by one trained physician researcher with the intra-reader correlation coefficient (ICC) of 0.81 and 0.84 for the NWI (coronal and sagittal readings) and 0.83 and 0.93 for β = intercondylar notch angle (coronal and sagittal).
Fig. 1.
Sagittal image with the Blumensaat’s line marking the roof of the intercondylar notch. The current reference cursor at the anterior outlet of the notch is represented by the beginning of the Blumensaat’s line. MR details: sagittal 3D DESS with water excitation, slice thickness of 0.7 mm, 16.3 ms TR, 4.7 ms TE, 25° flip angle, 14 cm × 14 cm FOV; 384 × 307 pixels matrix; 185 Hz/pixel bandwidth, 1 average, anterior/posterior phase encoding, acquisition time 10 min 23 s.
Fig. 2.
Measurements of the femoral notch on an axial image. The notch depth is perpendicular to a line connecting the ventral articular surfaces of the medial and the lateral femur condyle. The notch width and the condylar width are parallel to that line at 2/3 of the notch depth. MR details: axial reformatted (MPR) images of SAG 3D DESS: slice thickness of 0.7 mm, 16.3 ms TR, 4.7 ms TE, 25° flip angle, 14 cm × 14 cm FOV; 384 × 307 pixels matrix; 185 Hz/pixel bandwidth, 1 average, anterior/posterior phase encoding, acquisition time 10 min 23 s.
Fig. 3.
Measurements of the femoral notch on a coronal image. The notch depth is perpendicular to a line connecting the ventral articular surfaces of the medial and the lateral femur condyle. The notch width and the condylar width are parallel to that line at 2/3 of the notch depth. MR details: coronal IW TSE (COR_IW_TSE), slice thickness of 3 mm, 3850 ms TR, 29 ms TE, 180° FA, 14 cm × 14 cm FOV; 384 × 307 pixels matrix; 352 Hz/pixel bandwidth; acquisition time 3 min 24 s.
Osteophyte volume measurements
The analysis of the osteophytes volume using the DESS sequences was done within the segmentation process28 from a former study23, investigating cartilage morphometry changes in the same participants out of the OAI database. Therefore the following steps have been performed.
Bone segmentation: the bone boundaries were segmented by Virutal Scopics, using an automated approach. This was followed by a trained observer, who corrected segmentations errors and voxel miss-classification errors. The bones segmentations were used to extract data on cartilage morphometry. After this segmentation process, an expert radiologist verified the bone boundaries and the cartilage boundaries and made sure that all osteophytes have been labeled as bone tissue.
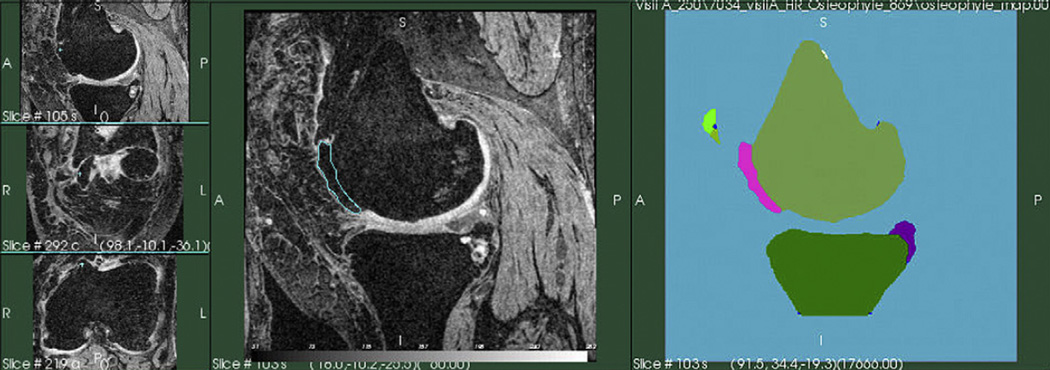
Osteophyte segmentation: the boundaries of the segmented bones areas were analyzed by mathematical morphology filtering, to detect all bone structures that do not match the expected smooth surface of the bone. All the bone elements, which were in the original segmentation and not present in the filtered bone segmentation were classified as possible osteophytes, as shown in Fig. 4. In a next step, all osteophyte candidates were divided into the areas of interest using the anatomical joint areas28. Osteophyte candidates at the shaft of the bones were not included. An expert radiologist supervised the osteophyte segmentation and removed all false positives. Tibia spine areas were the most common location for false positives.
Osteophyte volume computation: once the segmentations were reviewed, the osteophytes volumes were computed by aggregating all the voxels inside each osteophyte. For our analysis we used the osteophyte volume measured in the femur for adjustment.
Fig. 4.
Quantification of osteophytes. Central panel – coronal reconstructions of the DESS series showing the location of the detected osteophytes. Right panel – rendering of osteophytes. Image by Virtual Scopics.
STATISTICAL ANALYSIS
Descriptive statistics were performed, using Student’s t-tests for continuous variables and Chi-square tests for categorical variables. We examined whether notch stenosis was associated with an increased frequency of ACL tears. We also compared the mean values of NWI and notch angle between knees with ACL tears and those without ACL tears using a linear regression model. We then examined whether this relation was still present after adjusting for osteophyte volume and demographic variables, in those with and without injury, using multiple linear regression. Osteophyte bone volume was cubic root transformed to ensure a normal distribution. All statistical analyses were performed using SAS software, (SAS Institute Inc, Cary, North Carolina, release 8.2).
Results
The relation of ACL tear frequency with the NWI and the intercondylar notch angle from the measurements on coronal and axial sequences are shown in Tables II and III. On the coronal sequences, only two participants were categorized as having notch stenosis, using 0.20 as the critical value. No significant difference was shown in the frequency of femoral notch stenosis or smaller notch angle between participants with or without a torn ACL. The continuous measure of NWI showed a significant (P = 0.01) difference with participants with ACL tears having a narrower notch (mean NWI 0.246, 95% CI 0.234–0.258 mm) compared to those without an ACL tear (mean NWI 0.263, 95% CI 0.258–0.263 mm).
Table II.
Results from coronal sequences: frequency of ACL tear in participants with notch stenosis (NWI ≤ 0.2 and b < 50°) and NWI in participants with and without ACL tear (N = 160)
| ACL tear | P-value | ||
|---|---|---|---|
| Yes (N = 23) | No (N = 137) | ||
| Coronal | |||
| NWI ≤ 0.2 | 1 (4.4%) | 1 (0.7%) | 0.27 |
| NWI > 0.2 | 22 (95.6%) | 136 (99.3%) | |
| Intercondylar notch angle β < 50° | 11 (47.8%) | 56 (40.9%) | 0.53 |
| Intercondylar notch angle β ≥50° | 12 (52.2%) | 81 (59.1%) | |
| NWI mean and 95% CI | 0.246 ± 0.03 (0.234–0.258) | 0.263 ± 0.03 (0.258–0.268) | 0.01 |
| Intercondylar notch angle mean and 95% CI | 48.0 ± 8.1 (43.5–52.4) | 52.2 ± 11.2 (50.4–54.1) | 0.08 |
Table III.
Results from axial sequences: frequency of ACL tear in participants with notch stenosis (NWI ≤ 0.2 and b < 50() and NWI in participants with and without ACL tear (N = 160)
| ACL tear | P-value | ||
|---|---|---|---|
| Yes (N = 23) | No (N = 137) | ||
| Axial | |||
| NWI ≤ 0.2 | 17 (73.9%) | 90 (65.7%) | 0.44 |
| NWI > 0.2 | 6 (26.1%) | 47 (34.3%) | |
| Intercondylar notch angle β < 50° | 5 (21.7%) | 29 (21.2%) | 1.00 |
| Intercondylar notch angle β ≥50° | 18 (78.3%) | 108 (78.2%) | |
| NWI mean and 95% CI | 0.181 ± 0.03 (0.168–0.194) | 0.190 ± 0.03 (0.184–0.195) | 0.23 |
| Intercondylar notch angle mean and 95% CI | 55.3 ± 10.3 (50.9–59.8) | 58.5 ± 10.8 (56.7–60.3) | 0.19 |
The intercondylar notch angle as a continuous variable on the coronal images showed differences trending toward significance (P = 0.08). On the axial sequences there was no significant relation between measures of notch stenosis and ACL tears.
Data on osteophyte bone volume was available for 150 of the 160 examined participants and derived from MRI (mm3). The mean osteophyte volume was 1999.7 ± 2075.0 mm3 and ranged from 0.0 mm3 to 11,565.9 mm3. In 150 out of 160 patients we demonstrated that on the coronal images a significantly higher NWI in those participants with ACL tears (mean = 0.247, 95% CI 0.235–0.260) compared to those without ACL tears (mean = 0.262, 95% CI 0.258–0.267) was still present after adjusting for demographic variables including age, gender and BMI (P = 0.03) and still trending to significance after adjusting for demographic variables and femoral osteophyte volume.
On the axial images we could demonstrate a trend to significance for NWI after adjusting for demographic variables (P = 0.08). For the difference in the intercondylar notch angle measurements no significant results were found after adjusting for demographic variables or for osteophyte volume in addition, neither in the axial nor in the coronal images. (Tables IV and V).
Table IV.
Comparison of NWI and intercondylar notch angle between two ACL groups, adjusting for osteophyte bone volume of the posterior femur and demographic variables (N = 150) for coronal sequences
| Coronal | ACL (N = 20) Mean (95% CI) |
No ACL (N = 130) Mean (95% CI) |
P-value | |
|---|---|---|---|---|
| Adjusted for demographic | NWI | 0.247 (0.235–0.260) | 0.262 (0.258–0.267) | 0.03 |
| variables (age, gender) (N = 160) | Intercondylar notch angle | 48.90 (44.28–53.25) | 52.06 (50.23–53.89) | 0.22 |
| Adjusted for demographic | NWI | 0.248 (0.233–0.262) | 0.262 (0.257–0.268) | 0.06 |
| variables + osteophyte volume (cubic root transformed) (N = 150) | Intercondylar notch angle | 48.87 (43.62–54.12) | 51.62 (49.72–53.52) | 0.34 |
Table V.
Comparison of NWI and intercondylar notch angle between two ACL groups, adjusting for osteophyte bone volume of the posterior femur and demographic variables (N = 150) for axial sequences
| Axial | ACL (N = 20) Mean (95% CI) |
No ACL (N = 130) Mean (95% CI) |
P-value | |
|---|---|---|---|---|
| Adjusted for demographic | NWI | 0.177 (0.164–0.191) | 0.190 (0.185–0.196) | 0.08 |
| variables (age, gender) (N = 160) | Intercondylar notch angle | 55.24 (50.58–59.90) | 58.53 (56.69–60.37) | 0.20 |
| Adjusted for demographic | NWI | 0.181 (0.166–0.196) | 0.190) (0.184–0.195) | 0.32 |
| variables þosteophyte volume (cubic root transformed) (N = 150) | Intercondylar notch angle | 55.23 (49.90–60.57) | 58.35 (56.42–60.29) | 0.29 |
In a post-hoc analysis, we redefined femoral notch stenosis as the lowest 25% of the NWI values in our dataset, but were unable to find a statistical difference for the frequency of ACL tears. Further, the comparison of medians for NWI and femoral notch angle for participants with a value smaller than the mean for those two measurements did not reach a significant level.
Discussion
Using MRI, our study showed that the NWI in a population with knee OA, unselected for ACL tears, was significantly smaller in participants with ACL tear compared to participants with an intact ACL on the coronal images. Subjects with an ACL tear also showed a trend to a smaller intercondylar notch angle. We could not demonstrate an increased frequency of ACL tears when comparing participants with and without notch stenosis, using the critical values for NWI and notch angle proposed in the literature14,26,27.
Thus in a sample with predominant knee OA the NWI appears to be smaller in participants with ACL tears. Notch stenosis is known to contribute to pain and subjective knee instability, due to osteophytes that irritate the ACL or play a role in ACL integrity19. Prior studies have demonstrated that young patients with a preexisting notch stenosis are at a higher risk for ACL injuries14,29–34. These findings led to a number of recommendations and possible interventions, including physical examination, counseling, prophylactic knee bracing, sport restrictions31 and even prophylactic notchplasty35. However not all of these recommendations will play an important role in OA patients.
ACL injuries are known to accelerate and amplify the development of OA in the knee joint8–10,13,36–41 and may contribute to a specific pattern of disease progression20–22. Putting these two findings together, preexisting notch stenosis in knee OA may be identified as a factor influencing further disease progression and some of the recommendations described above may be applicable for clinical practice. Possible recommendations that could be applied or modified for OA patients might include prophylactic knee bracing, to prevent ACL tears in patients with a reduced notch width. Furthermore counseling regarding lifestyle changes that may increase the risk of ACL tears and restrictive recommendations regarding high risk sports may be adopted.
The NWI as a method to compare intercondylar notch widths was established through two radiographic studies16,27 by Souryal et al. Persons at higher risk for a contra-lateral ACL tears could be identified in those young individuals with low NWI and preexisting ACL injury from non contact maneuvers. In a second prospective study27, a normal range for the NWI and its correlation to ACL injuries was established.
In a prospective study including notch view radiographs from 415 knees of athletes LaPrade and Burnett32 also demonstrated a significant correlation between femoral notch stenosis and ACL tears. Fifteen percent of the athletes with a NWI ≤ 0.20 suffered from an ACL injury compared to 0.27% in those athletes with a normal NWI.
These well established radiographic methods served as basic principles for the development of our MRI measurements and we also adopted the normal values suggested by Souryal et al.16,27 for the NWI.
Applying the NWI for another imaging technique, the femoral notch of 48 young patients were examined in a case–control CT-study by Anderson et al.14. This study suggested a critical value of 0.2 for the NWI and 50° for the opening notch angle. The critical value for the notch angle we measured on MRI images was deduced from this study.
At the time of total knee replacement (TKR) Wada et al.18 observed the intercondylar notch areas of 32 patients suffering from severe medial compartment knee OA and 54 cadaver knees. Knees with more severe OA had a significantly smaller notch width than those with milder OA or normal knees. Furthermore a smaller notch width was correlated with ACL tears in the osteoarthritic knees. These finding regarding the association of ACL tears and a reduced notch width is consistent with what we found in our study. In this study sample none of the participants had a history of prior joint injury. Also the smaller notch width in the study by Wada et al.18 was thought to be a consequence of osteophyte growth in the intercondylar notch area. Therefore the explanation given is, that osteophyte growth in the femoral notch is affected by the function of the ACL and therefore may be accelerated by a dysfunctional or torn ACL. In contrast to this suggestion our finding was nearly irrespective of osteophyte volume, shown by the significant difference in the NWI that was still present after adjusting for femoral osteophyte volume.
In a radiographical study by Hernigou and Garabedian17, 30 osteoarthritic knees were evaluated by radiographs and CT. A smaller notch width and a smaller notch angle were associated with missing ACLs. The authors suggest therefore a narrower notch to increase the risk for deficiency of the ACL. Again the study did not provide any adjustment or measurements for osteophytes in the femoral notch, so that one can assume the association shown here is again explained similar to the one described by Wada et al.18.
The shape of the intercondylar notch was compared between osteoarthritic and non-osteoarthritic specimens in a study by Shepstone et al.42. In a sample of 96 human femora, they could demonstrate that OA seems to lead to a more straight shaped edge of the medial condyle compared to a concave shape in the non-osteoarthritic samples. The authors state a possible predisposing factor for the development of knee OA or a secondary remodeling. These findings support that there is a significant difference in the dimensions of the femoral notch between normal and osteoarthritic knees. It is not further distinguished in this study if the differences are due to the onset of OA or may have been present before.
Considering the studies described before, one should allow for a potential development patterns of femoral notch stenosis in knee OA. One would be the acquired notch narrowing, caused by osteophytes in the femoral notch, that lead to a progressive irritation and erosion of a former healthy ACL17,18,42. The second pathway of notch stenosis development would be a form, preexisting from youth that may lead to an ACL tear while doing competitive sports or play a role as a risk factor for an ACL tear under other influences, such as OA. In our study we tried to evaluate the association between the latter and ACL tears in OA, by minimizing the effect of acquired notch stenosis through adjusting for femoral osteophyte volume.
While our study sample was convenient and suitable, the rate of ACL tears was smaller than previously described in the literature with 13 (8.1%) complete ACL tears and 10 (6.25%) partial ACL tears and an overall tear rate of 14.4% in the whole study population. Three studies investigating ACL and other findings in knee OA presented rates from 22%7, over 28%6 to 35%5, for full thickness tears. However two of the studies with higher tear rates had a significantly smaller sample size which could explain the differences with our study. Additionally only 85% of the participants in our study had KLG ≥ 2, which may influence the discrepancy.
LIMITATIONS OF THIS STUDY
The elimination of the influence from secondary notch stenosis caused by osteophytes located in the femoral notch was minimized by adjusting the measurements for osteophyte volume. However osteophyte growth localized in the femoral notch could still bias the preexisting notch stenosis that we were trying to identify as a confounding factor for ACL tears. No distinction was made regarding the critical value for the NWI between male and female study participants as proposed in the original study27.
Furthermore the fact that 95.7% of the participants with an ACL tear reported a history of knee injury (Table I) constrained the feasibility of our second hypothesis. During the assessment of knee injury the question has been asked if the participants have ever injured their knee badly enough to have experienced a walking limitation for at least 2 days, which may not lead to the assumption that all these injuries had been badly enough to cause a traumatic ACL rupture which is known to be a typical injury in high impact sports.
The fidelity of MRI in ACL diagnosis has an accuracy between 90% and 100%43–46 compared to the gold standard method knee arthroscopy, but is yet to be demonstrated in patients with knee OA.
Due to the characteristics of this cross sectional study, our findings do not represent information about the hazard over time establishing preexisting notch stenosis as a risk factor for knee OA.
Conclusion
No prior studies have investigated MRI-based femoral notch measurements and ACL findings in a large cohort of osteoarthritic knees adjusting for femoral osteophyte volume. We could demonstrate that a smaller NWI is associated with ACL tears in persons with knee OA and therefore may serve as an independent confounder for ACL tears and therefore for the progression and the pattern of knee OA. Further research addressing other longitudinal influences on ACL tears in OA and determine how to best define femoral notch stenosis in persons with knee OA should be encouraged.
Acknowledgments
We would like to thank the Principal Investigators (Michael Nevitt, Kent Kwoh, Charles B. Eaton, Rebecca Jackson, Marc Hochberg, Joan Bathon), Co-Investigators and staff of the Osteoarthritis Initiative.
I would also like to thank the New Enlgand Baptist Hospital (NEBH) Research Funding Award Program for sponsoring my work with one of their Awards.
The OAI is a public–private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation. The Osteoarthritis Initiative and this pilot study are conducted and supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (N01-AR-2-2262, N01-AR-2-2262, and N01-AR-2-2258) in collaboration with the OAI Investigators and Consultants. This manuscript has been reviewed by the OAI Publication committee for scientific content and data interpretation.
Footnotes
Conflict of interest
No financial or personal relationships with organizations or other people are disclosed by any of the authors.
References
- 1.Spector TD, Hart DJ. How serious is knee osteoarthritis? Ann Rheum Dis. 1992;51(10):1105–1106. doi: 10.1136/ard.51.10.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 3.van Saase JL, van Romunde LK, Cats A, Vandenbroucke JP, Valkenburg HA. Epidemiology of osteoarthritis: Zoetermeer survey. Comparison of radiological osteoarthritis in a Dutch population with that in 10 other populations. Ann Rheum Dis. 1989;48(4):271–280. doi: 10.1136/ard.48.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunlop DD, Manheim LM, Song J, Chang RW. Arthritis prevalence and activity limitations in older adults. Arthritis Rheum. 2001;44(1):212–221. doi: 10.1002/1529-0131(200101)44:1<212::AID-ANR28>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 5.Chan WP, Lang P, Stevens MP, Sack K, Majumdar S, Stoller DW, et al. Osteoarthritis of the knee: comparison of radiography, CT, and MR imaging to assess extent and severity. AJR Am J Roentgenol. 1991;157(4):799–806. doi: 10.2214/ajr.157.4.1892040. [DOI] [PubMed] [Google Scholar]
- 6.Link TM, Steinbach LS, Ghosh S, Ries M, Lu Y, Lane N, et al. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology. 2003;226(2):373–381. doi: 10.1148/radiol.2262012190. [DOI] [PubMed] [Google Scholar]
- 7.Hill CL, Seo GS, Gale D, Totterman S, Gale ME, Felson DT. Cruciate ligament integrity in osteoarthritis of the knee. Arthritis Rheum. 2005;52(3):794–799. doi: 10.1002/art.20943. [DOI] [PubMed] [Google Scholar]
- 8.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63(3):269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDaniel WJ, Jr, Dameron TB., Jr The untreated anterior cruciate ligament rupture. Clin Orthop Relat Res. 1983;172:158–163. [PubMed] [Google Scholar]
- 10.Kannus P, Jarvinen M. Posttraumatic anterior cruciate ligament insufficiency as a cause of osteoarthritis in a knee joint. Clin Rheumatol. 1989;8(2):251–260. doi: 10.1007/BF02030082. [DOI] [PubMed] [Google Scholar]
- 11.Clatworthy M, Amendola A. The anterior cruciate ligament and arthritis. Clin Sports Med. 1999;18(1):173–198. doi: 10.1016/s0278-5919(05)70134-4. vii. [DOI] [PubMed] [Google Scholar]
- 12.Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage. 1995;3(4):261–267. doi: 10.1016/s1063-4584(05)80017-2. [DOI] [PubMed] [Google Scholar]
- 13.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 14.Anderson AF, Lipscomb AB, Liudahl KJ, Addlestone RB. Analysis of the intercondylar notch by computed tomography. Am J Sports Med. 1987;15(6):547–552. doi: 10.1177/036354658701500605. [DOI] [PubMed] [Google Scholar]
- 15.Shelbourne KD, Davis TJ, Klootwy kTE. The relationship between intercondylar notch width of the femur and the incidence of anterior cruciate ligament tears. A prospective study. Am J Sports Med. 1998;26(3):402–408. doi: 10.1177/03635465980260031001. [DOI] [PubMed] [Google Scholar]
- 16.Souryal TO, Moore HA, Evans JP. Bilaterality in anterior cruciate ligament injuries: associated intercondylar notch stenosis. Am J Sports Med. 1988;16(5):449–454. doi: 10.1177/036354658801600504. [DOI] [PubMed] [Google Scholar]
- 17.Hernigou P, Garabedian JM. Intercondylar notch width and the risk for anterior cruciate ligament rupture in the osteoarthritic knee: evaluation by plain radiography and CT scan. Knee. 2002;9(4):313–316. doi: 10.1016/s0968-0160(02)00053-4. [DOI] [PubMed] [Google Scholar]
- 18.Wada M, Tatsuo H, Baba H, Asamoto K, Nojyo Y. Femoral intercondylar notch measurements in osteoarthritic knees. Rheumatology (Oxford) 1999;38(6):554–558. doi: 10.1093/rheumatology/38.6.554. [DOI] [PubMed] [Google Scholar]
- 19.Leon HO, Blanco CE, Guthrie TB, Martinez OJ. Intercondylar notch stenosis in degenerative arthritis of the knee. Arthroscopy. 2005;21(3):294–302. doi: 10.1016/j.arthro.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Costa-Paz M, Muscolo DL, Ayerza M, Makino A, Aponte-Tinao L. Magnetic resonance imaging follow-up study of bone bruises associated with anterior cruciate ligament ruptures. Arthroscopy. 2001;17(5):445–449. doi: 10.1053/jars.2001.23581. [DOI] [PubMed] [Google Scholar]
- 21.Scarvell JM, Smith PN, Refshauge KM, Galloway HR, Woods KR. Association between abnormal kinematics and degenerative change in knees of people with chronic anterior cruciate ligament deficiency: a magnetic resonance imaging study. Aust J Physiother. 2005;51(4):233–240. doi: 10.1016/s0004-9514(05)70004-0. [DOI] [PubMed] [Google Scholar]
- 22.Scarvell JM, Smith PN, Refshauge KM, Galloway H, Woods K. Comparison of kinematics in the healthy and ACL injured knee using MRI. J Biomech. 2005;38(2):255–262. doi: 10.1016/j.jbiomech.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Hunter DJ, Niu J, Zhang Y, Totterman S, Tamez J, Dabrowski C, et al. Change in cartilage morphometry: a sample of the progression cohort of the Osteoarthritis Initiative. Ann Rheum Dis. 2008 doi: 10.1136/ard.2007.082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterfy C, Li J, Zaim S, Duryea J, Lynch J, Miaux Y, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test–retest reproducibility. Skeletal Radiol. 2003;32(3):128–132. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 25.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cha JH, Lee SH, Shin MJ, Choi BK, Bin SI. Relationship between mucoid hypertrophy of the anterior cruciate ligament (ACL) and morphologic change of the intercondylar notch: MRI and arthroscopy correlation. Skeletal Radiol. 2008;37(9):821–826. doi: 10.1007/s00256-008-0527-3. [DOI] [PubMed] [Google Scholar]
- 27.Souryal TO, Freeman TR. Intercondylar notch size and anterior cruciate ligament injuries in athletes. A prospective study. Am J Sports Med. 1993;21(4):535–539. doi: 10.1177/036354659302100410. [DOI] [PubMed] [Google Scholar]
- 28.Tamez-Pena J, Barbu-McInnis M, Lerner A, Totterman S. Unsupervised definition of the tibia-femoral joint regions of the human knee and its applications to cartilage analysis (Abstract) 2006 [Google Scholar]
- 29.Davis TJ, Shelbourne KD, Klootwyk TE. Correlation of the intercondylar notch width of the femur to the width of the anterior and posterior cruciate ligaments. Knee Surg Sports Traumatol Arthrosc. 1999;7(4):209–214. doi: 10.1007/s001670050150. [DOI] [PubMed] [Google Scholar]
- 30.Good L, Odensten M, Gillquist J. Intercondylar notch measurements with special reference to anterior cruciate ligament surgery. Clin Orthop Relat Res. 1991;263:185–189. [PubMed] [Google Scholar]
- 31.Houseworth SW, Mauro VJ, Mellon BA, Kieffer DA. The intercondylar notch in acute tears of the anterior cruciate ligament: a computer graphics study. Am J Sports Med. 1987;15(3):221–224. doi: 10.1177/036354658701500305. [DOI] [PubMed] [Google Scholar]
- 32.LaPrade RF, Burnett QM. Femoral intercondylar notch stenosis and correlation to anterior cruciate ligament injuries. A prospective study. Am J Sports Med. 1994;22(2):198–202. doi: 10.1177/036354659402200208. [DOI] [PubMed] [Google Scholar]
- 33.Lund-Hanssen H, Gannon J, Engebretsen L, Holen KJ, Anda S, Vatten L. Intercondylar notch width and the risk for anterior cruciate ligament rupture. A case–control study in 46 female handball players. Acta Orthop Scand. 1994;65(5):529–532. doi: 10.3109/17453679409000907. [DOI] [PubMed] [Google Scholar]
- 34.Palmer I. On the injuries to the ligaments of the knee joint: a clinical study. 1938. Clin Orthop Relat Res. 2007;454:17–22. doi: 10.1097/BLO.0b013e31802c7915. [DOI] [PubMed] [Google Scholar]
- 35.Teitz CC, Lind BK, Sacks BM. Symmetry of the femoral notch width index. Am J Sports Med. 1997;25(5):687–690. doi: 10.1177/036354659702500517. [DOI] [PubMed] [Google Scholar]
- 36.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 37.Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22(5):632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 38.Fithian DC, Paxton EW, Stone ML, Luetzow WF, Csintalan RP, Phelan D, et al. Prospective trial of a treatment algorithm for the management of the anterior cruciate ligament-injured knee. Am J Sports Med. 2005;33(3):335–346. doi: 10.1177/0363546504269590. [DOI] [PubMed] [Google Scholar]
- 39.Meunier A, Odensten M, Good L. Long-term results after primary repair or non-surgical treatment of anterior cruciate ligament rupture: a randomized study with a 15-year follow-up. Scand J Med Sci Sports. 2007;17(3):230–237. doi: 10.1111/j.1600-0838.2006.00547.x. [DOI] [PubMed] [Google Scholar]
- 40.Segawa H, Omori G, Koga Y. Long-term results of non-operative treatment of anterior cruciate ligament injury. Knee. 2001;8(1):5–11. doi: 10.1016/s0968-0160(00)00062-4. [DOI] [PubMed] [Google Scholar]
- 41.Wilder FV, Hall BJ, Barrett JP, Jr, Lemrow NB. History of acute knee injury and osteoarthritis of the knee: a prospective epidemiological assessment. The Clearwater Osteoarthritis Study. Osteoarthritis Cartilage. 2002;10(8):611–616. doi: 10.1053/joca.2002.0795. [DOI] [PubMed] [Google Scholar]
- 42.Shepstone L, Rogers J, Kirwan JR, Silverman BW. Shape of the intercondylar notch of the human femur: a comparison of osteoarthritic and non-osteoarthritic bones from a skeletal sample. Ann Rheum Dis. 2001;60(10):968–973. doi: 10.1136/ard.60.10.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JK, Yao L, Phelps CT, Wirth CR, Czajka J, Lozman J. Anterior cruciate ligament tears: MR imaging compared with arthroscopy and clinical tests. Radiology. 1988;166(3):861–864. doi: 10.1148/radiology.166.3.3340785. [DOI] [PubMed] [Google Scholar]
- 44.Rose NE, Gold SM. A comparison of accuracy between clinical examination and magnetic resonance imaging in the diagnosis of meniscal and anterior cruciate ligament tears. Arthroscopy. 1996;12(4):398–405. doi: 10.1016/s0749-8063(96)90032-8. [DOI] [PubMed] [Google Scholar]
- 45.Glashow JL, Katz R, Schneider M, Scott WN. Double-blind assessment of the value of magnetic resonance imaging in the diagnosis of anterior cruciate and meniscal lesions. J Bone Joint Surg Am. 1989;71(1):113–119. [PubMed] [Google Scholar]
- 46.Polly DW, Jr, Callaghan JJ, Sikes RA, McCabe JM, McMahon K, Savory CG. The accuracy of selective magnetic resonance imaging compared with the findings of arthroscopy of the knee. J Bone Joint Surg Am. 1988;70(2):192–198. [PubMed] [Google Scholar]