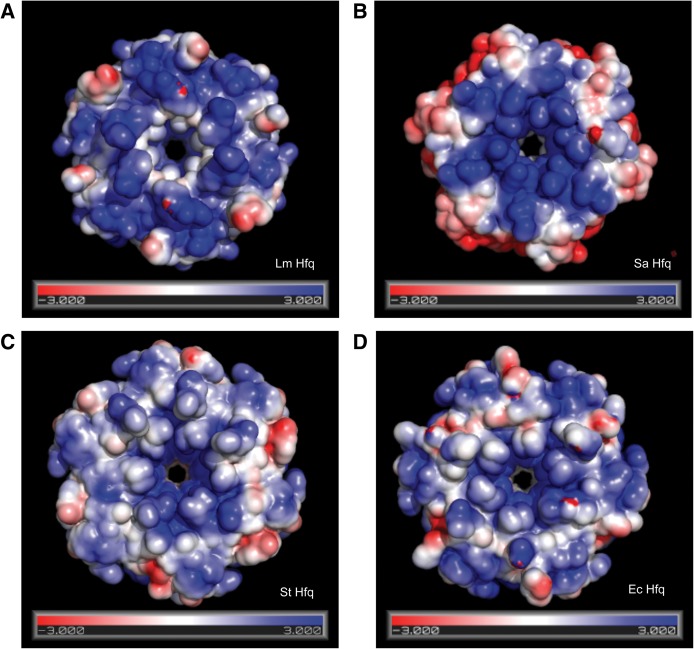
FIGURE 3.
Surface electrostatic potentials of selected Hfq proteins. Views of the surface electrostatic potentials of the proximal faces of (A) Lm Hfq, (B) Sa Hfq (PDB: 1KQ1), (C) St Hfq (PDB:2YLB), and (D) Ec Hfq (PDB:1HK9), contoured at ±3 kT/e (T = 310 K). Calculations were made using the APBS plug-in in PyMol (Baker et al. 2001). N.B.: The pore of Lm Hfq, the U6 RNA binding location, is less positive than the other three Hfq proteins. The electrostatic surface of the proximal pore of each protein likely has a strong influence on the binding affinity of each protein for U6 RNA.