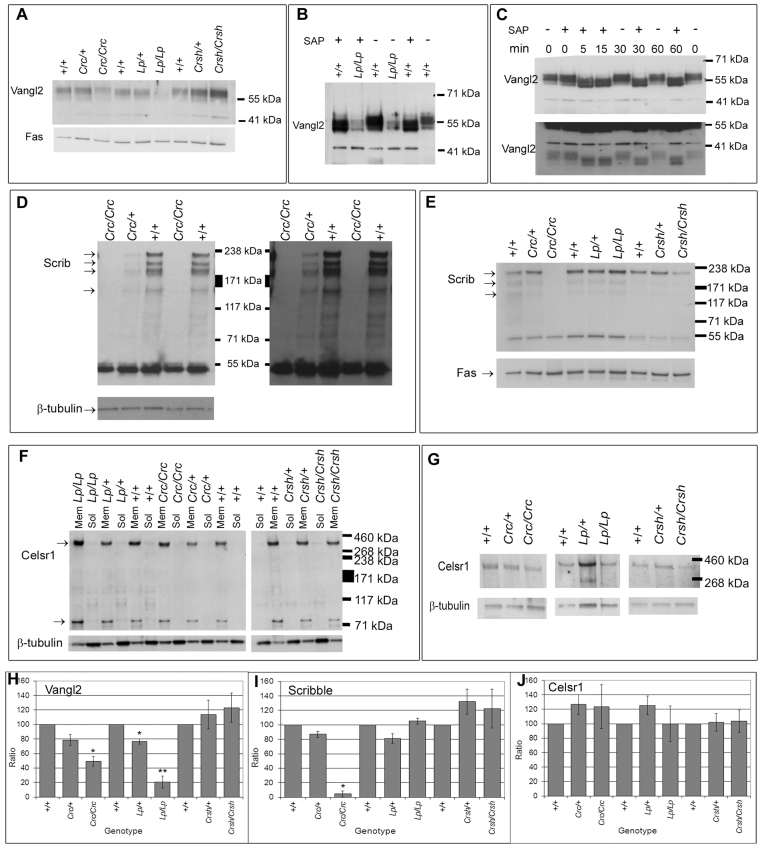
Fig. 5.
Protein analysis suggests that ScribCrc is a null mutant, whereas Vangl2Lp and Celsr1Crsh express mutant protein isoforms. Western blot analysis was performed on whole embryo lysates (unless otherwise stated) using antibodies specific for the individual PCP proteins. (A) Vangl2 protein expression in E8.5 wild-type, heterozygous and homozygous embryos for ScribCrc, Vangl2Lp and Celsr1Crsh. Vangl2 has reduced abundance in Vangl2Lp/Lp and to a lesser extent in ScribCrc embryos. Fatty acid synthase (Fas) was used as loading control. (B) Vangl2 protein expression in membrane fractions from E10.5 wild-type and Vangl2Lp/Lp embryos, following incubation with (+) or without (−) shrimp alkaline phosphatase (SAP). Vangl2Lp/Lp mutants show reduced Vangl2 expression, and SAP treatment causes a shift in band sizes in wild type consistent with decreased phosphorylation in Vangl2Lp/Lp mutants. (C) Vangl2 protein expression in wild-type E11.5 membrane and organelle fraction, treated with (+) or without (−) SAP, for times from 0 to 60 minutes as indicated above each lane. SAP incubation causes a shift in band distribution to a lower molecular weight, although three bands can still be seen (upper panel). This suggests that Vangl2 is phosphorylated endogenously, but that other modifications are also present, giving rise to the multiple bands observed. Extended exposure reveals bands at lower molecular weights (lower panel), indicating the existence of shorter isoforms (or cleavage products), that might correspond to alternative splice variants predicted on Ensembl. (D) Scrib protein expression in membrane fractions of E13.5 wild-type, ScribCrc/+ and ScribCrc/Crc fetuses. A duplicate blot was immunostained for β-tubulin. Scrib is detected as multiple isoforms from ~210 kDa (arrows). ScribCrc/Crc mutants completely lack Scrib protein, with no evidence for stably expressed truncated product, even after extended exposures (right-hand panel). The monoclonal antibody detects a band at ~55 kDa, in both wild-type and ScribCrc/Crc mutants; no known splice variants are predicted to generate a shorter isoform of this size, and this band seems likely the result of nonspecific antibody binding. (E) Scrib expression in E8.5 wild-type, heterozygous and homozygous embryos from ScribCrc, Vangl2Lp and Celsr1Crsh litters. Scrib is absent from ScribCrc/Crc embryos. The lower molecular weight bands varied in intensity between protein preparations and could be either degradation products of Scrib protein or alternatively spliced isoforms. (F,G) Celsr1 protein expression in membrane (Mem) and soluble (Sol) fractions of E10.5 embryos (F) and whole-cell lysates of E8.5 embryos (G) from Vangl2Lp, ScribCrc and Celsr1Crsh litters. Celsr1 bands (arrows in F) are detected at ~350 kDa (F,G) and ~80 kDa (F), with no apparent change in any mutants. (H–J) Quantitation of Vangl2 (H), Scrib (I) and Celsr1 (J) protein expression in E8.5 ScribCrc, Vangl2Lp and Celsr1Crsh embryos, normalized to β-tubulin (H) or Fas (I,J) levels. Data are the average of three experiments ± standard errors; expression levels that differ significantly from wild type are indicated with asterisks (*P<0.05, **P<0.01).