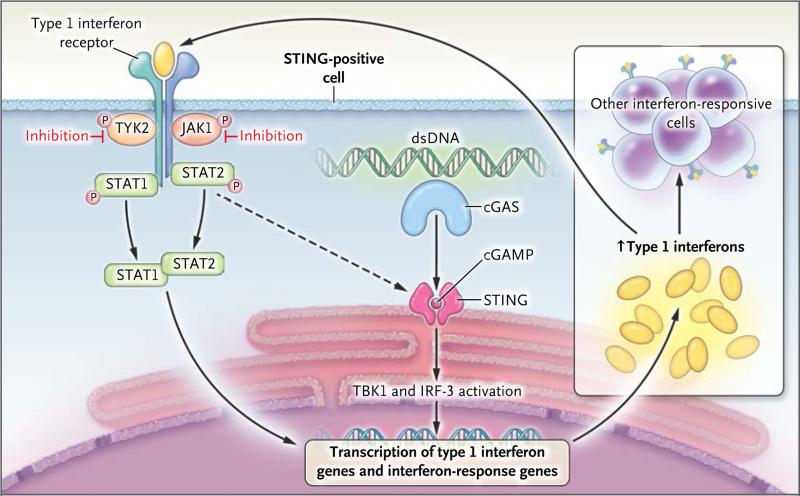
Figure 3. The STING–Interferon-β Pathway.
STING, an endoplasmic reticulum transmembrane protein, forms homodimers and functions as an adaptor for cytosolic DNA sensing. STING is activated by the binding of cyclic guanosine monophosphate–adenosine monophosphate (cGAMP), a second messenger that is synthesized by cyclic GMP–AMP synthase (cGAS), a family member of nucleotidyltransferases that is activated on its recognition and binding of double-stranded DNA (dsDNA). Binding of cGAMP to the STING homodimer activates interferon regulatory factor 3 (IRF-3) through TANK-binding kinase 1 (TBK1) and leads to the induction of interferon-β. In patients with SAVI, constitutively activated STING leads to increased transcription of the type 1 interferon gene, IFNB1, which encodes interferon-β. Binding of inter feron-β to its receptor activates Janus kinases (JAKs), including JAK1 and tyrosine kinase 2 (TYK2), which in turn phosphory-late the receptor. This process allows the binding of the DNA-binding proteins signal transducers and activators of transcription 1 (STAT1) and 2 (STAT2) to the receptor, whereupon they become phosphorylated (P). Phosphorylation allows them to dimerize, and the dimer translocates to the nucleus, where it up-regulates transcription of interferon-response genes, including interferon regulatory factor 7–dependent transcription of type 1 interferon genes. The synthesis and release of interferons and their binding to interferon receptor further up-regulate STING and the transcription of other proinflammatory cytokine genes in a positive feedback loop. JAK inhibition blocks the loop, resulting in a decrease in STAT1 phosphorylation and transcription of its target genes in vitro.