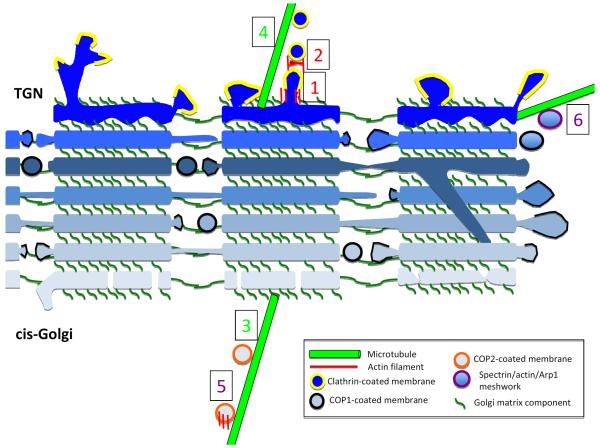
Figure 6. Possible roles for actin and microtubules in Golgi function.
Schematic cross-section of three laterally-connected mini-stacks from mammalian Golgi*, with individual cisternae in blue (lightest are cis- and darkest are TGN). Golgi matrix proteins (dark green) tether cisternae vertically within a mini-stack, and horizontally between mini-stacks. A sub-set of parallel cisternae establish lateral membrane connections, which are likely to be highly dynamic. Occasionally, inter-cisternae membrane connections might occur within a mini-stack (one depicted here). COP1-mediated vesicle transport (black banding around membrane) occurs from the edges of mini-stacks. COP2-coated vesicles arrive at cis-Golgi via ERGIC. At the TGN, exiting transport membranes are generally clathrin-coated (yellow banding). From our interpretation of the literature, we postulate six known or highly likely instances of cytoskeletal involvement in Golgi dynamics, some being actin-dependent (red numbers), some microtubule-dependent (green numbers) and some involving both actin and microtubules (purple numbers). (1) tubulation of TGN, involving actin, Arp2/3 complex and myosin 1B. (2) fission of transport vesicles from TGN, involving actin and myosin II. (3) transport of COP2 vesicles to cis-Golgi-attached minus ends of microtubules by dynein. (4) transport of clathrin vesicles away from TGN by kinesins. (5) transport of tubulovesicular membranes from ERGIC to cis-Golgi, involving actin, microtubules and WHAMM. (6) Spectrin coating of Golgi-derived membranes, possibly containing actin and/or Arp1, and interacting with microtubule motors in an as-yet un-defined manner.