Abstract
We explored the effect of a novel synthetic triterpenoid compound Cyano Enone of Methyl Boswellates (CEMB) on various prostate cancer and glioma cancer cell lines. CEMB displayed concentration-dependent cytotoxic activity with submicromolar lethal dose 50% (LD50) values in ten of ten tumor cell lines tested. CEMB-induced cytotoxicity is accompanied by activation of downstream effector caspases (caspases 3 and 7) and by upstream initiator caspases involved in both the extrinsic (caspase 8) and intrinsic (caspase 9) apoptotic pathways. By using small interfering RNAs (siRNAs), we show evidence that knock down of caspase 8, death receptor 4 (DR4), Apaf-1, and Bid impairs CEMB-induced cell death. Similar to other proapoptotic synthetic triterpenoid compounds, CEMB-induced apoptosis involved endoplasmic reticulum (ER) stress, as demonstrated by partial rescue of tumor cells by siRNA-mediated knock-down of expression of genes involve in the unfolded protein response such as Ire1, Perk, and ATF6. Altogether our results suggest that CEMB stimulates several apoptotic pathways in cancer cells, suggesting that this compound should be evaluated further as a potential agent for cancer therapy.
Keywords: Triterpenoid, Apoptosis, ER stress
Introduction
Synthetic triterpenoid compounds are a class of anticancer agents possessing beneficial activities. Thus far, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid (CDDO) and its derivative compounds CDDO-Me, CDDO-Im are the most potent triterpenoid compounds known in terms of anti-inflammatory and anti-carcinogenic activity (1). These compounds have anti-proliferative activity against cancer cells, anti-inflammatory activity against mouse macrophages, and pro-apoptotic activity against various types of cancers and leukemias, as well as inducing differentiation of neuronal cell lines and preadipocytes (2). Among the many types of cancer and leukemia cells where CDDO class triterpenoids have been reported to induce apoptosis are B and T cell leukemias (3–6), breast cancers (including both ER positive and negative) (7, 8), ovarian cancers (9), prostate cancers (10, 11), pancreatic carcinomas (12), various skin cancers (13) and lung cancers (14–17). In addition, CDDO, CDDO-Me and CDDO-Im have been shown to inhibit tumour growth in xenograft models in mice and rats (8, 10, 18). Currently, CDDO-Me is in phase I clinical trials as a novel cancer therapeutic agent.
The cytotoxic mechanism of triterpenoids is multifunctional. Recent studies suggest that CDDO and its derivatives induce Reactive Oxygen Species (ROS) (12, 19), decrease mitochondrial glutathione (12, 19, 20), activate JNK and p38 MAPK stress pathways (14, 21–23), and inhibit NF-κB activity (24, 25).
The triterpenoid classes of anticancer and bioactive natural products are electrophiles (26). Recently, these electrophilic compounds were shown to trigger ER stress (27), presumably by interfering with disulfide-dependent folding of proteins in the ER. The accumulation of unfolded proteins in the ER stimulates signal transduction events that include activation of the ER transmembrane kinases Ire1 and Perk, as well as proteolytic processing and activation of the transcription factor ATF6 (reviewed in (28). Downstream components of the unfolded protein response (UPR) are capable of activating the two major apoptotic pathways – the extrinsic cell death pathway activated by TNF-family ligands and death receptors and the intrinsic cell death pathway initiated by release of apoptogenic proteins from mitochondria (29);Kurokawa, 2009 #17465;Kim, 2008 #15839]. In several studies, it has been shown that the CDDO and its derivatives induce cell death either through intrinsic (30–32) or extrinsic pathways (14, 16, 33). Some studies indicate that both extrinsic and intrinsic pathways are involved depending on the cell type (10). CDDO-Me also induces ER stress, including CHOP expression, TRAIL receptor expression, and caspase-dependent killing of tumor cells (27). Thus, ER, mitochondrial, and death receptor pathways are all activated by CDDO and its active analogs.
As CDDO and its derivatives have been recognized as promising agents for treatment of cancers, we investigated the synthesis and characterization of additional types of synthetic triterpenoid compounds. In this report, we investigated the cytotoxic mechanism of CEMB, a closely related analogue of CDDO (Supplemental Figure 1). In our previous work, we synthesized triterpenoid compounds and demonstrated that the cyano enone of methyl boswellate (CEMB), arjunolic acid, and glycyrrhetinic acid inhibit inflammation in primary mouse macrophages stimulated by interferon-γ and induce cell death of some cancer cell lines (34). Among the three synthetic triterpenoids that we tested, the most bioactive was CEMB, which was extracted and modified from Boswellia Serata. The parent structure of CDDO belongs to the olean skeleton while that of CEMB is a mixture of ursane and olean skeletons – the two isomeric skeletons differ only in the position of the methyl group on the E-ring. Both CDDO and CEMB share the cyano-enone moiety at the A-ring of the structures. The differences between the two structures are in the position of the carboxylate moiety and the functionality of the C-ring. While CDDO has the polar carboxylate moiety on the E-ring, CEMB carries it as a pendant of the A-ring. Furthermore CDDO has an enone functionality in the C-ring, while CEMB retains the unsaturated C-ring in the parent skeleton. Since the synthesis of CEMB does not involve the chemical modifications involved in the functionalization of the C-ring, the number of steps required for its synthesis is reduced in comparision to CDDO. In this study, we have investigated the in vitro activity of CEMB against prostate cancer and glioma cancer cells, and we have explored the mechanism by which CEMB induces apoptosis.
Materials and Methods
Reagents
The triterpenoid compound, CEMB, was synthesized from boswellic acid. For a detailed protocol describing synthesis of CEMB, refer to our earlier work (34). The CEMB compound was dissolved in DMSO at the concentration of 10 mM and aliquots were stored at −80°C. Stock compound was diluted in culture medium just before cell treatment. The ATPlite 1-step luminescence assay kit was purchased from Perkin Elmer. The Caspase-Glo 3/7 assay, Caspase-Glo 9 and Caspase-Glo 8 assay kits were purchased from Promega. RNAiMAX was obtained from Invitrogen. The OptiMEM was purchased from GIBCO. RPMI and DMEM media came from Mediatech Inc. San Diego, CA. Pre-designed small interfering RNAs (siRNAs) directed against caspase 8 (SiRNA IDs: 9572, 9478, 9663), DR4 (SiRNA IDs: 202067, 4816, 5004), DR5 (SiRNA Ids: 107249, 5003), and Bid (SiRNA Ids: 120775, 120774, 2556, 120776) were purchased from Ambion. Apaf-1 siRNAs (SiRNA Ids: SI02662387, SI02661876, SI02654428) were obtained from Qiagen. TRAIL was obtained from Genentech (South San Francisco, California). Primers for IRE1α 5′-3′-(F-ggcctggtcaccacaattag; R-gggtcagcactgtcctctgt), PERK (F-gctgtatccattcagcactcag; R-gcatgtcttgaaccatcacg), ATF6 (F-gcagctggatgaagttgtgt; R-ccaacatgctcataggtcca), and Ask1 (F-cctgaagcttaagtcccaacc; R-cattcactctcagccagtcg) were designed and obtained from Valuegene Inc., San Diego. CHOP-Luc plasmid was kindly provided by Prof. Pierre Fafournoux, France.
Cell lines and culture conditions
Prostate cancer cell lines (PPC-1, Alva-31, DU145, LNCAP, and PC3) and glioma cell lines (U87, U343, U251, LN229 and C6) were obtained from American Type Culture Collection (ATCC). The cell lines have not been recently tested and authenticated. Prostate cancer cells were cultured in RPMI and glioma cells were cultured in DMEM supplemented with 10% fetal bovine serum (GIBCO) and maintained at 37°C in a humidified atmosphere containing 5% CO2 and 95% air.
Cell viability assays
MTT and ATPlite assays were used for cell viability estimation. For MTT assay, cells were seeded at a density of 5 × 103 cells/well in 100 μl of culture medium in 96-well flat-bottom plates and cultured overnight. The next day, compound treatment was carried out by adding various concentrations of compounds directly to the wells followed by incubation for 72 hours. Then, 20 μl of MTT (5 mg/ml) was added to each well and plates were returned to the incubator for 3 hours. The supernatant medium was removed and the dark blue crystals that had formed were dissolved in 200 μl of DMSO, followed by reading absorbance at 550 nm. For ATP assays, where ATP serves as a surrogate for cell viability, we employed the ATPlite 1-step luminescence assay (Perkin-Elmer). Cells were plated at a density of 2 × 103 cells/well in 45 μL complete medium in 384-well solid white plates and cultured overnight. The next day, cells were cultured with various concentrations of compounds for various times (5 μL). Plates were then removed from the incubator and allowed to equilibrate to room temperature for about 10 minutes. ATPlite solution was added 25 μl per well and plates were kept in dark for 5 minutes prior to reading luminescence using a luminometer (Luminoskan Ascent, Therom Electron Corporation).
Caspase activity assays
Prostate cancer and glioma cells were plated (2 × 103 cells/well) in 384-well solid white bottom plates and cultured overnight in 45 μL of complete medium. The next day, compounds were added directly to the wells (5 μL) and cells were incubated for 6, 12, 24 or 48 hours. Caspase Glo-3/7, Caspase Glo-8, or Caspase Glo-9 substrate was added (50 μL) and the plates were returned to the incubator. After 30 minutes to 1 hour of incubation, luminescence was measured.
Silencing of genes using small interfering RNA (siRNA)
PPC-1 cells were transfected with the siRNAs directed against caspase 8, DR4, DR5, Bid, Apaf-1, Ire1α, Perk, ATF6, or Ask1 for 48 hours by a reverse transfection method using Lipofectamine RNAiMAX, according to the manufacturer’s instructions (Invitrogen). For each gene, up to 4 different siRNAs sequences were used. After 48 hours of transfection, cells were treated with various compounds, typically for 24 hours. Cell viability assays were performed as described above. To confirm the silencing of genes by the siRNA, total protein or RNA was isolated after transfection and analyzed by either immunoblotting or by RT-PCR. The detailed protocol is given (see below).
Immunoblot analysis
PPC-1 cells were plated at the density of 3 × 105 cells/well in 6-well plates and cultured for overnight. The next day, cells were treated with 1–2 μM CEMB for 24 hours. Cells were trypsinized, washed with PBS, and lysed with RIPA buffer (50 mM Tris [pH7.4], 150 mM NaCl, 1 mM EDTA, 0.1% Na-deoxycholate, 1% Triton X100, and protease inhibitors). Protein concentrations were quantified by Bradford’s assay. Samples were normalized for total protein content, loading 50 μg to 100 μg per lane for SDS-PAGE/immunoblot analysis.
RNA isolation and analysis
PPC-1 cells (3.50×105 cells/well) were plated in 6 wells plate and transfected with siRNAs for 48 hours by using RNAiMAX. Cells were trypsinized, washed with PBS, and the cell pellets were collected for RNA isolation. To determine RNA levels after compound treatments, PPC-1 cells were plated in 6 well dishes at the density of 0.5×106 cells/well and next day treated with compounds for 3h, 6h, 12h, and 24h. After the treatment, cells were washed with PBS, trypsinized, and pelleted. QIAGEN RNAeasy mini kit was used to extract RNA according to the manufacturer’s instructions.
Plasmid DNA transfection
PPC-1 cells were plated in 96 well plates at density of 2×104 cells/well in 50 μL of culture medium. After 6h, 3.2 μg of CHOP-Luc plasmid was introduced into the cells along with 0.07 μg of Renilla-Luc, as an internal control for transfection using lipofectamine 2000 in 50 μL of OptiMEM. The next day, medium was removed and fresh medium was added along with various compounds for 10h. To terminate assays, medium was removed and cells were lysed with 1x “passive lysis buffer” (Promega) for 15 minutes at room temperature on a rocking platform. The Dual Glo-luciferase assay kit (Promega) was used to measure the firefly/Renilla luciferase ratio.
Results
CEMB displays potent cytotoxic activity against prostate cancer and glioma cell lines
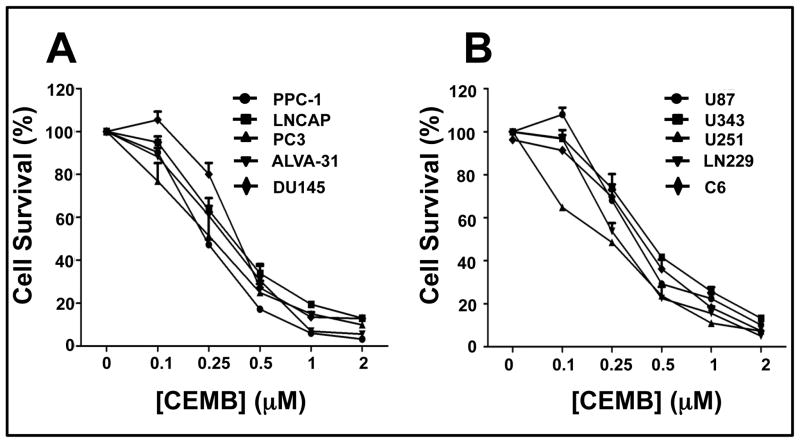
Because the synthetic triterpenoid CDDO is reported to have single-agent apoptotic activity against prostate cancer and glioblastoma cell lines (10, 25), we tested CEMB for cytotoxic activity against several such tumor cell lines in culture (n = 5 each). Three days of treatment with various concentrations of CEMB, ranging from 0.1 μM to 2 μM, resulted in dose-dependent cell death of all ten tumor cell lines with submicromolar lethal dose 50% (LD50) concentrations as measured by MTT assay (Figures 1a and b). Results were confirmed by measuring cellular ATP levels in CEMB-treated cells, again with submicromolar LD50 values of all ten tumor cell lines (Supplementary data: Tables 1 and 2).
Figure 1. CEMB exhibits concentration-dependent cytotoxic activity against prostate cancer and glioma cell lines.
(A) Prostate cancer cell lines, PPC1, LNCAP, PC3, ALVA-31 and DU145, and (B) glioma cell lines, U87, U343, U251, LN229 and C6, were treated with CEMB for 72 hours and then assayed for cell death using MTT. The x-axis denotes the CEMB concentration (in μM) and the y-axis denotes relative cell survival (% compared to control treated with DMSO) as measured by MTT assay. All the experiments were performed in quadruplicate (mean ± SEM). An initial cell density of 5,000 cells/well of 96 well plates was used, adding compounds after 24 hours.
CEMB-induced cytotoxicity is partially caspase dependent
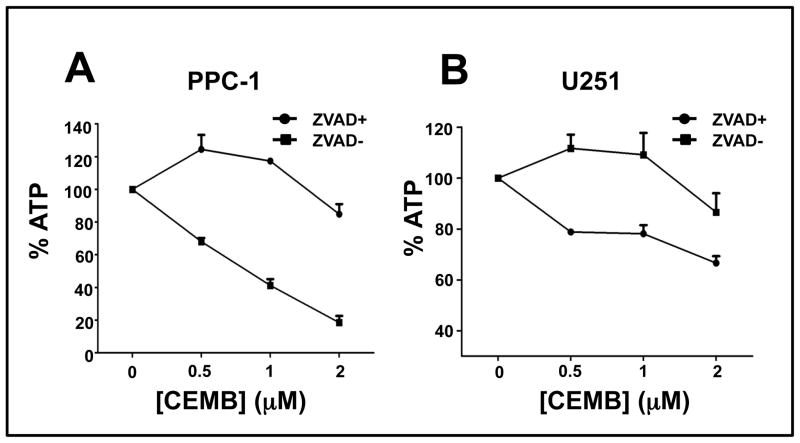
We used the cell-permeable pan-caspase inhibitor zVAD-fmk to test the caspase dependence of CEMB induced killing of PPC-1 prostate and U251 glioblastoma cells. For these experiments, tumor cells were treated with 100 μM of zVAD-fmk and various concentrations of CEMB. CEMB-induced cell death was significantly suppressed by zVAD-fmk in cultures of both PPC-1 and U251 cells as measured by the surrogate indicator ATP levels (Figures 2a and 3b) and by trypan blue dye exclusion (not shown).
Figure 2. CEMB-induced cell death is partly caspase dependent.
Prostate cancer cell line PPC-1 (A) or glioma cell line U251 (B) were plated in a 96 well plates and treated with (circle) or without (square) z-VAD-fmk (100 μM) for 2 hours, and then treated with CEMB at various concentrations. After 48 hours, cellular ATP levels were measured. The x-axis is the CEMB concentration (in μM) and y-axis is the relative level of ATP, expressed as % relative to cells cultured with DMSO. Values are the average of independent measurements. (bars, SE)
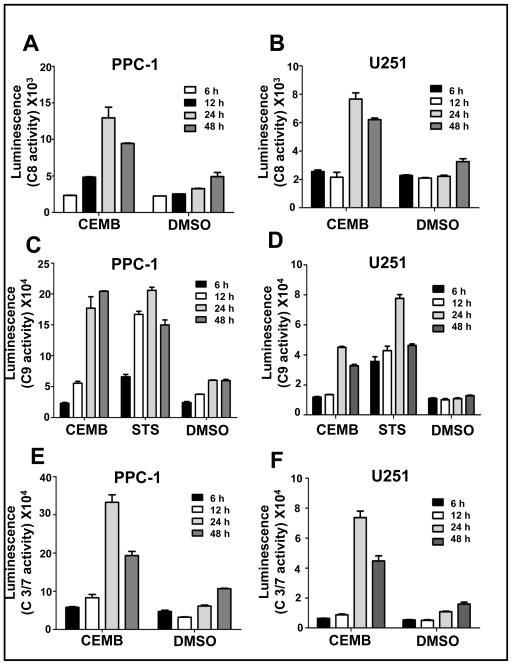
Figure 3. Activation of caspases by CEMB.
PPC-1 (A, C, E) or U251 cells (B, D, F) were plated in 384-white plates (102 cells/well) and next day treated with CEMB for 6, 12, 24, 48 hours. Relative levels of activities of caspase 8, 9 and 3/7 were measured using fluorigenic substrates. The x-axis represents the respective compounds used, namely CEMB (2 μM) and Staurosporine (1 μM) and DMSO (vehicle control). The y-axis denotes the luminescence of the corresponding caspases measured by Glo-caspase 8, Glo-caspase 9 and Glo-caspase 3/7 substrates. The experiment was repeated 2 or 3 times. Data presented here are results from one of the experiments where assays were performed in triplicate (mean ± SEM; n=3).
CEMB activates caspases
Activation of caspase 8 and caspase 9 plays key roles in the extrinsic and intrinsic apoptosis pathways, respectively. We measured the activities of the upstream initiator proteases caspase 8 (extrinsic pathway) and caspase 9 (intrinsic pathway), as well as downstream executioner proteases caspases 3 and 7 using fluorigenic substrates that are preferrentially hydrolyzed by these proteases. Staurosporine was used as a positive control for caspase-9 activation, as this broad-spectrum protein kinase inhibitors is known to cause apoptosis via the intrinsic apoptotic pathway (35). CEMB treatment resulted in increases in caspase 8, caspase 9 and caspase 3/7 activity, with maximal activity occurring typically at approximately 24 hours post-treatment (Figure 3). Consistent with these results, CEMB also induced proteolytic processing of pro-caspase-8, pro-caspase-9, and PARP (supplemental Figure 2). Altogether, these findings suggest that CEMB activates both the intrinsic and extrinsic apoptosis pathways.
CEMB-induced apoptosis of PPC-1 cells requires caspase-8, Trail Receptor, Bid, and Apaf-1
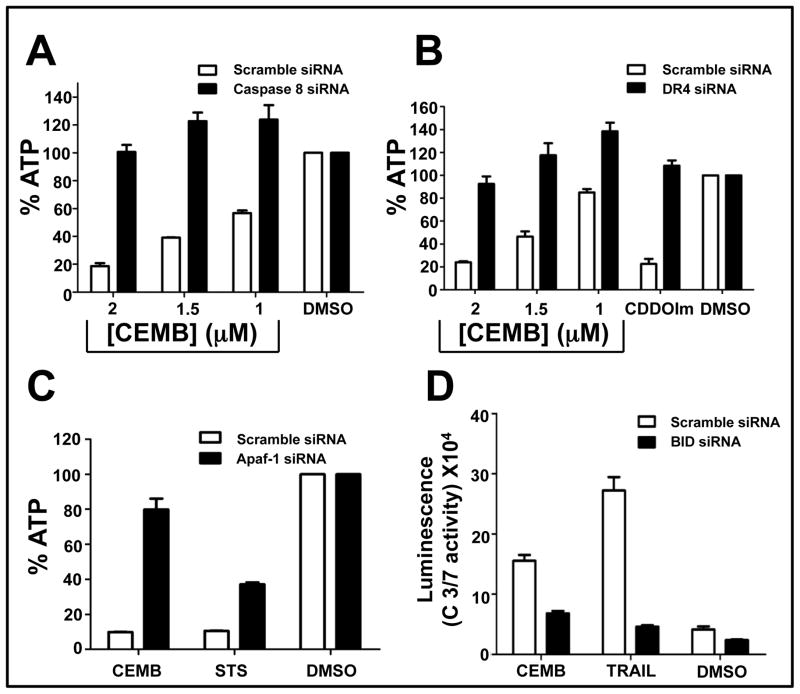
We used siRNA to investigate some of the apoptosis pathway components involved in CEMB-induced apoptosis in the PPC-1 prostate cancer cell line. Each siRNA used here was confirmed to reduce expression of the corresponding target mRNA (supplemental Figures 3 and 4), including extrinsic pathway components caspase-8 and DR4, and the intrinsic pathway components Bid and Apaf-1. Scrambled sequence siRNAs served as negative controls. Note that we were unable to successfully knock-down caspase-9, despite testing 4 different siRNA sequences, and the efficacy of siRNAs targeting DR5 mRNA was variable, thus precluding definitive conclusions (not shown). In addition to CEMB, cells were treated with either TRAIL or STS as controls for extrinsic and intrinsic pathway agonists, respectively. Cell viability was assessed by ATP measurements.
CEMB-induced killing of PPC-1 cells was suppressed by caspase 8 and DR4 siRNAs (Figures 4a, b), implying that this compound stimulates an apoptotic mechanism involving the TRAIL receptor DR4 in these prostate cancer cells. The siRNAs targeting Bid and Apaf-1 also markedly suppressed CEMB-induced cytotoxicity (Figures 4c, d), implying involvement of the intrinsic pathway in the cytotoxic mechanism of CEMB in PPC-1 cells. Results were independently confirmed by assessing apoptosis by morphological inspection of DAPI-stained cells (not shown).
Figure 4. Caspase 8, DR4, Apaf-1 and Bid are required for CEMB-induced cell death.
PPC-1 or DU145 cells were cultured in 384-well plates and transfected with caspase 8 (A), DR4 (B) or Afaf-1 (C) siRNAs. After 48 hours, cells were treated with CEMB (various concentrations), TRAIL (100 ng) or CDDO-Im (1.5 μM) for 24 hours. ATP levels were measured, expressing data as % relative to DMSO control (mean ± SEM; n=3). PPC-1 cells (D) were transfected with Bid siRNAs for 48 hours followed by treatment with CEMB (1.5 μM), STS (0.25 μM) or TRAIL (100 ng) for 6 hours. Glo-caspase 3/7 activity assay was performed. The x-axis denotes the compounds. The y-axis denoted the Glo Caspase 3/7 luminescence readings. (mean ± SEM [n=3]).
CEMB-induced cytotoxicity involves components of the ER stress machinery
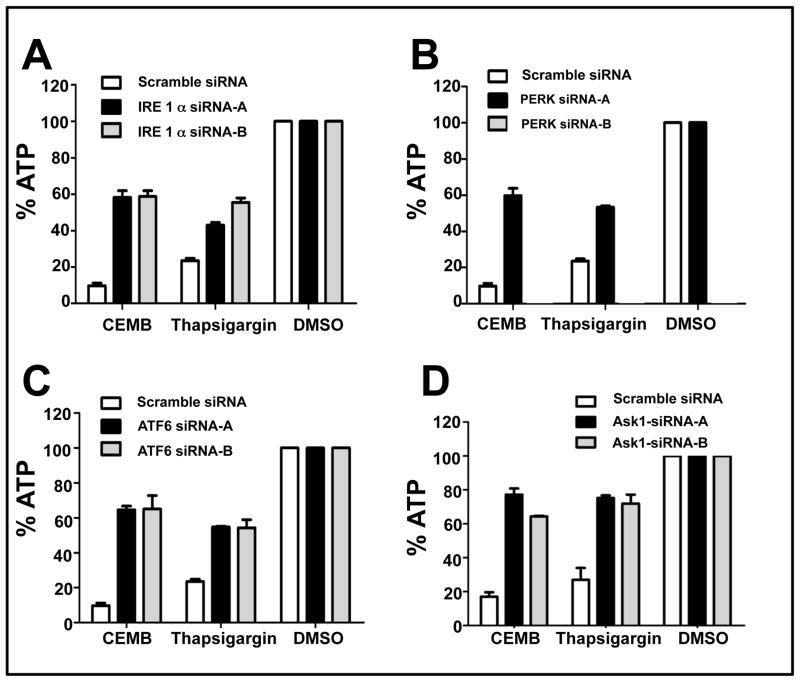
Because CDDO-Me has been reported to induce apoptosis through an ER stress mechanism (27), we investigated the role of UPR signaling molecules in the cytotoxic mechanism induced by CEMB. For these experiments, siRNAs targeting Ire1α, Ask1 (an apoptotic kinase downstream of Ire1α), Perk, and ATF6, as well as corresponding scrambled sequences (as negative controls) were introduced into PPC-1 cells by lipofection. At least two active siRNA sequences were identified for each target, and verified by RT-PCR to reduce target mRNA levels by > 60% (supplemental Figures 3 and 4). Comparisons were made of the ability of these siRNAs to reduce cell killing (measured by ATP) induced by CEMB with thapsigargin (THAP), an irreversible inhibitor of the ER Ca2+ ATPase (a known inducer of ER stress).
CEMB-induced cytotoxicity of PPC-1 cells was significantly reduced by all of the UPR components tested – Ire1α, Ask1, Perk, and ATF6 (Figure 5). These siRNAs also partially rescued PPC-1 cells from THPS-induced cytotoxicity, consistent with a role in ER stress. We also observed that CEMB induced modest increases (3- to 4- fold) in Ire1α and Perk mRNAs but not ATF6 or Ask1 (supplemental Figure 5).
Figure 5. siRNAs targeting ER stress pathway protect against CEMB-induced cytotoxicity.
PPC-1 cells were transfected with IRE1-α (A), PERK (B), ATF6 (C), or Ask1(D) siRNAs for 48 hours and then treated with CEMB (2 μM) or Thapsigargin (5 μM) for 24h. ATP levels were measured and data expressed as % relative to DMSO treated cells transfected with scrambled control siRNA (mean ± SEM; n=3).
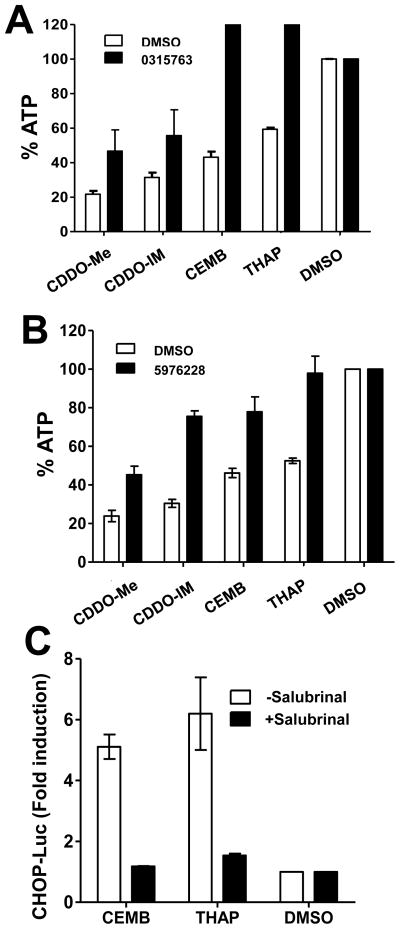
Next, we used small molecule inhibitors of ER stress induced cell death to interrogate the sole of ER stress in CEMB-mediated cytotoxicity. Benzodiazepinones such as CID-2891837 inhibit ER stress-induced apoptosis downstream of Ire1α, while Salubrinal suppresses UPR signalling downstream of Perk (36, 37). Both an analog of CID-2891837 and Salubrinal (Supplemental Figure 1) reduced CEMB-induced cytotoxicity, as well as killing induced by triterpenoids, CDDO-Me and CDDO-Im, and by ER-stress-inducer thapsigargin (Figure 6). These results obtained using chemical inhibitors of ER-stress-induced apoptosis. Thus provide further evidence that CEMB kills tumor cells at least in part through an ER-initiated cell death mechanism.
Figure 6. Chemical inhibitors of ER stress protect against CEMB-mediated cytotoxicity.
Tumor cells were cultured with 10 μM of benzodiazepinone MLS-0315763 (A), Salubrinal (B), or DMSO, then cultured with CEMB, CDDO analogs, or thapsigargin for 48hrs. Relative cell survival was measured by ATP assay as described above (mean ± SD; n=3) (C). To measure CHOP gene promotion activation, PPC-1 cells were transfected with CHOP-Luc reporter gene plasmid and Renilla-Luc normalization plasmid. The next day, cells were treated with (black bars) or without (white bars) Salubrinal for 1 hr, then treated with DMSO, CEMB, or Thapsigargin for 6h. The ratio of firefly:renilla luciferase activity was measured, expressing results as fold induction relative to cells treated with DMSO alone (mean ± SEM; n=3).
Finally, we assessed the effects of CEMB on UPR signalling event induced by the convergence of the Ire1α, Perk, and ATF6 pathways – namely, activation of the CHOP gene promoter, CHOP encodes a transcription factor that induces apoptosis by modulating expression of the BCL-2, BIM, DR5, and other apoptosis genes (38).
To measure CHOP gene activation after CEMB treatment, we transfected CHOP-Luc reporter gene plasmid to PPC-1 cells. As shown, CHOP gene promoter activity increased ~ 4-fold at 10h after treatment with CEMB, similar to Thapsigargin (Figure 6c). However, when PPC-1 cells were treated with Salubrinal, a compound that blocks ER stress signaling in the PERK pathway (36), then CHOP promoter activity was not induced. We conclude that CEMB is an inducer of ER stress and UPR signalling, and that CEMB mediates its cytotoxic actions at least in part through an ER stress mechanism.
Discussion
Triterpenoid compound boswellic acid has been used for treatment of inflammatory diseases such as arthritis and inflammatory bowel disease since ancient time in Indian medicine, particularly in ayurveda (39). In the present study, we have evaluated the cytotoxic activity of CEMB on tumor cell lines finding that CEMB as a single agent has potent in vitro cytotoxic activity against prostate cancer and glioblastoma cell lines. Most chemotherapeutic drugs induce apoptosis by the intrinsic apoptosis pathway involving mitochondria. Our present study showed that early apoptotic events such as caspase 8 and caspase 9 activation appear to be induced by CEMB treatment, suggesting that both extrinsic and intrinsic pathways are involved. Rescue of PPC-1 cells from CEMB by caspase-8 siRNA indicates a functionally important role for the extrinsic pathway, which is further supported by results of DR4 knock-down as well as Bid knock-down (a connector of the extrinsic pathway to the intrinsic pathway via caspase-8-mediated cleavage and activation of Bid (40). We also observed a role for the intrinsic apoptosis pathway, in that Apaf-1 knock-down rescued PPC-1 cells from CEMB. Unfortunately, we were unable to achieve Caspase-9 mRNA knock-down to further confirm the involvement of this pathway. Thus, our data suggest that at least in the tumor cell lines evaluated here, CEMB causes cell death by activating extrinsic and intrinsic pathways of apoptosis, possibly by first activating caspase-8 to result in Bid activation and then causing mitochondrial involvement. It should be noted that activation of proapoptotic Bcl-2 family members such as Bid is well known to cause mitochondrial outer membrane permeabilization (MOMP), which induces in parallel rapidly acting caspase-dependent and slower acting caspase-independent cytotoxic mechanisms (reviewed in (29)). Thus, killing by CEMB is likely to be only partly caspase dependent. Because chemoresistant cancers tend to have defects in the intrinsic mitochondrial pathway, synthetic triterpenoids such as CEMB may provide an alternative route to successfully inducing apoptosis of refractory cancer cells.
Based on experiments using siRNAs targeting proximal components of the ER stress/UPR machinery, as well as chemical inhibitors of ER stress signalling, we determined that the cytotoxic mechanism of CEMB involves, at least in part, the ER stress pathway. Previous studies have documented an important role for ER stress in the cytotoxic mechanism of other triterpenoids, such as CDDO analogs (27). The electrophilic nature of CEMB and other triterpenoid compounds presumably explains why they induce ER stress. The mechanisms linking ER stress to apoptosis are diverse (38). Included among the ER stress inducers of apoptosis is induction of CHOP, a transcription factor that stimulates expression of TRAIL receptors and that modulates expression of Bcl-2 family genes (38). We observed stimulation of CHOP gene promoter activity following treatment with CEMB. Because the ER stress response is capable of stimulating both intrinsic and extrinsic apoptosis pathways, the dominant pathway will likely depend on the specific tumor cell line or cancer type. Also, ER stress-initiated cell death mechanisms typically are only partly caspase dependent (reviewed in (38)), presumably explaining why siRNA mediated knock down of Apaf1 only partially rescued cells from CEMB-mediated cytotoxicity.
In summary, compared to CDDO and its anlalogs, CEMB is a more easily synthesized triterpenoid compound showing potent cytotoxic activity against tumor cell lines in culture and displaying a cytotoxic mechanism of action similar to CDDO-family compounds, including inducing ER stress and evidence of activation of both intrinsic and extrinsic apoptosis pathways. The relative merits of CEMB compared to CDDO analogs currently in clinical testing with respect to pharmacology and toxicology remain to be determined. Cytotoxic activity of CEMB against cultured hepatocytes was similar to CDDO-Im tested at concentration ≤ 1 μM (not shown). Further mechanistic studies and evaluations of CEMB in pre-clinical models will help to define the overall potential of CEMB as a novel therapeutic agent for cancer.
Supplementary Material
Acknowledgments
This work was supported by the NIH to J.C.R. (P01-CA-113318) and the DoD (W81XWH-08-0574). We thank M. Hanaii and T. Siegfried for manuscript preparation.
Abbreviations
- CEMB
References
- 1.Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007;7:357–69. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- 2.Suh N, Wang Y, Honda T, Gribble GW, Dmitrovsky E, Hickey WF, et al. A novel synthetic oleanane triterpenoid, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, with potent differentiating, antiproliferative, and anti-inflammatory activity. Cancer Res. 1999;59:336–41. [PubMed] [Google Scholar]
- 3.Ray DM, Morse KM, Hilchey SP, Garcia TM, Felgar RE, Maggirwar SB, et al. The novel triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO) induces apoptosis of human diffuse large B-cell lymphoma cells through a peroxisome proliferator-activated receptor gamma-independent pathway. Exp Hematol. 2006;34:1202–11. doi: 10.1016/j.exphem.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 4.Konopleva M, Tsao T, Ruvolo P, Stiouf I, Estrov Z, Leysath CE, et al. Novel triterpenoid CDDO-Me is a potent inducer of apoptosis and differentiation in acute myelogenous leukemia. Blood. 2002;99:326–35. doi: 10.1182/blood.v99.1.326. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen IM, Kitada S, Schimmer A, Kim Y, Zapata JM, Charboneau L, et al. The triterpenoid CDDO induces apoptosis in refractory CLL B-cells. Blood. 2002;100:2965–72. doi: 10.1182/blood-2002-04-1174. [DOI] [PubMed] [Google Scholar]
- 6.Suh W-S, Kim Y, Schimmer AD, Kitada S, Minden MD, Andreeff M, et al. Synthetic triterpenoids activate a pathway for apoptosis in AML cells involving down-regulation of FLIP and sensitization to TRAIL. Leukemia. 2003;17:2122–9. doi: 10.1038/sj.leu.2403112. [DOI] [PubMed] [Google Scholar]
- 7.Lapillonne H, Konopleva M, Tsao T, Gold D, McQueen T, Sutherland RL, et al. Activation of peroxisome proliferator-activated receptor gamma by a novel synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid induces growth arrest and apoptosis in breast cancer cells. Cancer Res. 2003;63:5926–39. [PubMed] [Google Scholar]
- 8.Hyer ML, Croxton R, Krajewska M, Krajewski S, Kress CL, Lu M, et al. Synthetic triterpenoids cooperate with tumor necrosis factor-related apoptosis-inducing ligand to induce apoptosis of breast cancer cells. Cancer Res. 2005;65:4799–808. doi: 10.1158/0008-5472.CAN-04-3319. [DOI] [PubMed] [Google Scholar]
- 9.Petronelli A, Saulle E, Pasquini L, Petrucci E, Mariani G, Biffoni M, et al. High sensitivity of ovarian cancer cells to the synthetic triterpenoid CDDO-Imidazolide. Cancer Lett. 2009;282:214–28. doi: 10.1016/j.canlet.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Hyer ML, Shi R, Krajewska M, Meyer C, Lebedeva IV, Fisher PB, et al. Apoptotic activity and mechanism of 2-cyano-3,12-dioxoolean-1,9-dien-28-oic-acid and related synthetic triterpenoids in prostate cancer. Cancer Res. 2008;68:2927–33. doi: 10.1158/0008-5472.CAN-07-5759. [DOI] [PubMed] [Google Scholar]
- 11.Vene R, Larghero P, Arena G, Sporn MB, Albini A, Tosetti F. Glycogen synthase kinase 3beta regulates cell death induced by synthetic triterpenoids. Cancer Res. 2008;68:6987–96. doi: 10.1158/0008-5472.CAN-07-6362. [DOI] [PubMed] [Google Scholar]
- 12.Samudio I, Konopleva M, Hail N, Jr, Shi YX, McQueen T, Hsu T, et al. 2-Cyano-3,12-dioxooleana-1,9-dien-28-imidazolide (CDDO-Im) directly targets mitochondrial glutathione to induce apoptosis in pancreatic cancer. J Biol Chem. 2005;280:36273–82. doi: 10.1074/jbc.M507518200. [DOI] [PubMed] [Google Scholar]
- 13.Hail N, Jr, Konopleva M, Sporn M, Lotan R, Andreeff M. Evidence supporting a role for calcium in apoptosis induction by the synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO) J Biol Chem. 2004;279:11179–87. doi: 10.1074/jbc.M312758200. [DOI] [PubMed] [Google Scholar]
- 14.Zou W, Liu X, Yue P, Zhou Z, Sporn MB, Lotan R, et al. c-Jun NH2-terminal kinase-mediated up-regulation of death receptor 5 contributes to induction of apoptosis by the novel synthetic triterpenoid methyl-2-cyano-3,12-dioxooleana-1, 9-dien-28-oate in human lung cancer cells. Cancer Res. 2004;64:7570–8. doi: 10.1158/0008-5472.CAN-04-1238. [DOI] [PubMed] [Google Scholar]
- 15.Liby K, Black CC, Royce DB, Williams CR, Risingsong R, Yore MM, et al. The rexinoid LG100268 and the synthetic triterpenoid CDDO-methyl amide are more potent than erlotinib for prevention of mouse lung carcinogenesis. Mol Cancer Ther. 2008;7:1251–7. doi: 10.1158/1535-7163.MCT-08-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou W, Chen S, Liu X, Yue P, Sporn MB, Khuri FR, et al. c-FLIP downregulation contributes to apoptosis induction by the novel synthetic triterpenoid methyl-2-cyano-3, 12-dioxooleana-1, 9-dien-28-oate (CDDO-Me) in human lung cancer cells. Cancer Biol Ther. 2007;6:1614–20. doi: 10.4161/cbt.6.10.4763. [DOI] [PubMed] [Google Scholar]
- 17.Liby K, Voong N, Williams CR, Risingsong R, Royce DB, Honda T, et al. The synthetic triterpenoid CDDO-Imidazolide suppresses STAT phosphorylation and induces apoptosis in myeloma and lung cancer cells. Clin Cancer Res. 2006;12:4288–93. doi: 10.1158/1078-0432.CCR-06-0215. [DOI] [PubMed] [Google Scholar]
- 18.Place AE, Suh N, Williams CR, Risingsong R, Honda T, Honda Y, et al. The novel synthetic triterpenoid, CDDO-imidazolide, inhibits inflammatory response and tumor growth in vivo. Clin Cancer Res. 2003;9:2798–806. [PubMed] [Google Scholar]
- 19.Ikeda T, Nakata Y, Kimura F, Sato K, Anderson K, Motoyoshi K, et al. Induction of redox imbalance and apoptosis in multiple myeloma cells by the novel triterpenoid 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid. Mol Cancer Ther. 2004;3:39–45. [PubMed] [Google Scholar]
- 20.Yue P, Zhou Z, Khuri FR, Sun SY. Depletion of intracellular glutathione contributes to JNK-mediated death receptor 5 upregulation and apoptosis induction by the novel synthetic triterpenoid methyl-2-cyano-3, 12-dioxooleana-1, 9-dien-28-oate (CDDO-Me) Cancer Biol Ther. 2006;5:492–7. doi: 10.4161/cbt.5.5.2565. [DOI] [PubMed] [Google Scholar]
- 21.Konopleva M, Contractor R, Kurinna SM, Chen W, Andreeff M, Ruvolo PP. The novel triterpenoid CDDO-Me suppresses MAPK pathways and promotes p38 activation in acute myeloid leukemia cells. Leukemia. 2005;19:1350–4. doi: 10.1038/sj.leu.2403828. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda T, Kimura F, Nakata Y, Sato K, Ogura K, Motoyoshi K, et al. Triterpenoid CDDO-Im downregulates PML/RARalpha expression in acute promyelocytic leukemia cells. Cell Death Differ. 2005;12:523–31. doi: 10.1038/sj.cdd.4401574. [DOI] [PubMed] [Google Scholar]
- 23.Stadheim TA, Suh N, Ganju N, Sporn M, Eastman A. The novel triterpenoid, 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO), potentlyenhances apoptosis induced by tumor necrosis factor in human leukemia cells. J Biol Chem. 2002 doi: 10.1074/jbc.M108974200. In Press. [DOI] [PubMed] [Google Scholar]
- 24.Shishodia S, Sethi G, Konopleva M, Andreeff M, Aggarwal BB. A synthetic triterpenoid, CDDO-Me, inhibits IkappaBalpha kinase and enhances apoptosis induced by TNF and chemotherapeutic agents through down-regulation of expression of nuclear factor kappaB-regulated gene products in human leukemic cells. Clin Cancer Res. 2006;12:1828–38. doi: 10.1158/1078-0432.CCR-05-2044. [DOI] [PubMed] [Google Scholar]
- 25.Gao X, Deeb D, Jiang H, Liu Y, Dulchavsky SA, Gautam SC. Synthetic triterpenoids inhibit growth and induce apoptosis in human glioblastoma and neuroblastoma cells through inhibition of prosurvival Akt, NF-kappaB and Notch1 signaling. J Neurooncol. 2007;84:147–57. doi: 10.1007/s11060-007-9364-9. [DOI] [PubMed] [Google Scholar]
- 26.Liby K, Hock T, Yore MM, Suh N, Place AE, Risingsong R, et al. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res. 2005;65:4789–98. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- 27.Zou W, Yue P, Khuri FR, Sun SY. Coupling of endoplasmic reticulum stress to CDDO-Me-induced up-regulation of death receptor 5 via a CHOP-dependent mechanism involving JNK activation. Cancer Res. 2008;68:7484–92. doi: 10.1158/0008-5472.CAN-08-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu C, Bailly-Maitre B, Reed JC. Endoplamic reticulum stress: Cell life and death decisions. J Clinical Invest. 2005;115:2656–64. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed JC. Mechanisms of apoptosis. Am J Pathol. 2000;157:1415–30. doi: 10.1016/S0002-9440(10)64779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brookes PS, Morse K, Ray D, Tompkins A, Young SM, Hilchey S, et al. The triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid and its derivatives elicit human lymphoid cell apoptosis through a novel pathway involving the unregulated mitochondrial permeability transition pore. Cancer Res. 2007;67:1793–802. doi: 10.1158/0008-5472.CAN-06-2678. [DOI] [PubMed] [Google Scholar]
- 31.Samudio I, Konopleva M, Pelicano H, Huang P, Frolova O, Bornmann W, et al. A novel mechanism of action of methyl-2-cyano-3,12 dioxoolean-1,9 diene-28-oate: direct permeabilization of the inner mitochondrial membrane to inhibit electron transport and induce apoptosis. Mol Pharmacol. 2006;69:1182–93. doi: 10.1124/mol.105.018051. [DOI] [PubMed] [Google Scholar]
- 32.Inoue S, Snowden RT, Dyer MJ, Cohen GM. CDDO induces apoptosis via the intrinsic pathway in lymphoid cells. Leukemia. 2004;18:948–52. doi: 10.1038/sj.leu.2403328. [DOI] [PubMed] [Google Scholar]
- 33.Ito Y, Pandey P, Sporn MB, Datta R, Kharbanda S, Kufe D. The novel triterpenoid CDDO induced apoptosis and differentiation of human osteosarcoma cells by a Caspase-8 dependent mechanism. Mol Pharmacol. 2001;59:1094–9. doi: 10.1124/mol.59.5.1094. [DOI] [PubMed] [Google Scholar]
- 34.Subba Rao GS, Kondaiah P, Singh SK, Ravanan P, Sporn MB. Chemical modifications of natural triterpenes - glycyrrhetinic and boswellic acids: evaluation of their biological activity. Tetrahedron. 2008;64:11541–8. doi: 10.1016/j.tet.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuyama S, Llopis J, Deveraux QL, Tsien RY, Reed JC. Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat Cell Biol. 2000;2:318–25. doi: 10.1038/35014006. [DOI] [PubMed] [Google Scholar]
- 36.Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–9. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 37.Kim I, Shu CW, Xu W, Shiau CW, Grant D, Vasile S, et al. Chemical Biology Investigation of Cell Death Pathways Activated by Endoplasmic Reticulum Stress Reveals Cytoprotective Modulators of ASK1. J Biol Chem. 2009;284:1593–603. doi: 10.1074/jbc.M807308200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–30. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 39.Gautam R, Jachak SM. Recent developments in anti-inflammatory natural products. Med Res Rev. 2009;29:767–820. doi: 10.1002/med.20156. [DOI] [PubMed] [Google Scholar]
- 40.Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death & Differ. 2000;7:1166–73. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.