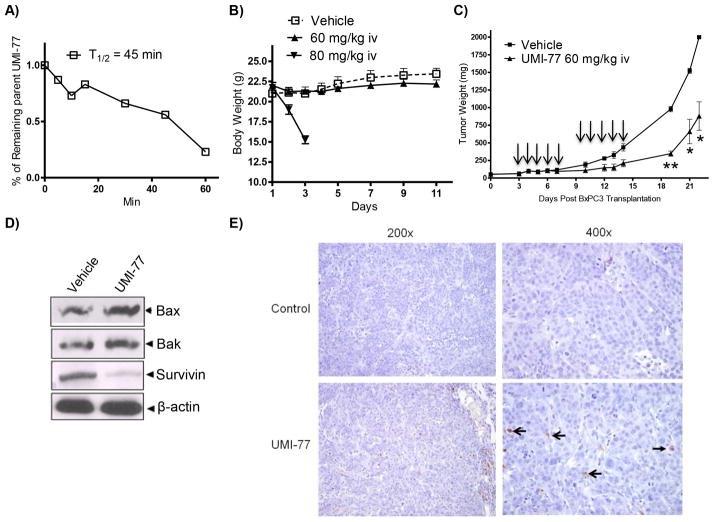
Fig. 5. In vivo characterization of 2 (UMI-77).
A) Determination of the microsome stability of UMI-77 (expressed as T1/2). B) Evaluation of the effect of UMI-77 on weight loss of SCID mice. UMI-77 was administrated two cycles i.v. for 5 days per week in two tested concentrations 60 and 80 mg/kg. C) In vivo efficacy of UMI-77 in BxPC-3 xenograft animal model. BxPC-3 xenografts were inoculated subcutaneously in SCID mice. Once transplanted, fragments developed into palpable tumors, about 60 mg, groups of 4 animals with bi-lateral tumors were removed randomly and assigned to two treatment groups. Mice were administered UMI-77 i.v 60 mg/kg for 5 consecutive days a week for two weeks. [■] vehicle treated group and [▲] UMI-77 treated groups. UMI-77 treated groups showed significant reduction of the tumor growth when compared to treatments with vehicle on day 19 (p < 0.0001), 20 (p < 0.003) and 21 (p < 0.003). D) Western blots analysis for different pro-apoptotic and survival markers on lysates isolated from tumours harvested from mice of different treatment groups showing enhancement of pro-apoptotic Bax and Bak and down-regulation of survivin compared with control. E) Immunohistochemical staining of BxPC-3 tumor xenografts using Apoptag Kit. No positively-staining nuclei are present in the control samples. Several positively-staining cells (open arrows) are present, as is apoptotic debris (closed arrows) in presented field (400× original magnification).