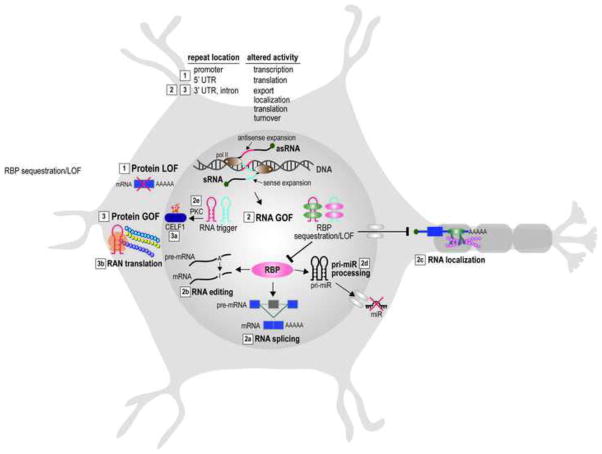
Fig. 1.
Neuronal cell model illustrating pathogenic effects of non-coding microsatellite expansions in neurologic disease. Both sense (blue line) and antisense (red line) DNA repeat expansions result in three major downstream deleterious effects (labeled 1–3): (1) protein loss-of-function (LOF) results from hypermethylation of a CG-rich expanded microsatellite repeat in the 5′ UTR and/or promoter region of an affected gene and loss of transcriptional activity (e.g., FXS); (2) RNA gain-of-function (GOF) occurs following bidirectional transcription of expanded repeats and the synthesis of sense (sRNA) and antisense (asRNA), which fold into RNA hairpins (sRNA, blue; asRNA, red) or other stable structures and gain toxic functions either by sequestering an RNA binding protein(s) (RBP) and inhibiting pre-mRNA splicing (2a), pre-mRNA editing (2b), mRNA localization (2c), pri-miR processing (2d) or by triggering aberrant cellular activities such as protein kinase C (PKC) mediated CELF1 hyperphosphorylation (2e); (3) protein GOF due to altered post-translation modifications of other RNA binding proteins (3a) (CELF1 hyperphosphorylation, purple oval with white P in orange star) or RAN translation (3b) (three homopolymeric repeat proteins are shown with one undergoing translation by the ribosome (orange). In addition to these mechanisms, other pathways, including chromatin remodeling, proteome dysregulation and vesicular trafficking, may also contribute to disease pathogenesis, particularly in the CNS (Hernandez-Hernandez et al., 2013).