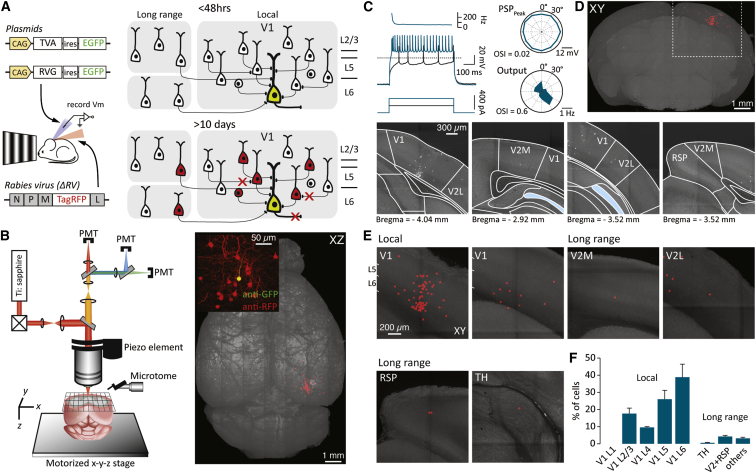
Figure 5.
Mapping Connectivity onto Individual CC Cells
(A) During whole-cell recording, the cell was loaded with DNA plasmids to drive expression of the rabies glycoprotein (RVG) and the avian virus receptor (TVA). This was followed by injection of the modified rabies virus (ΔRV) into the local area that results in targeted infection of the recorded neuron and subsequent retrograde spread and expression of RV-RFP.
(B) After at least 10 days postrecording, the brain was fixed and placed under a serial two-photon microscope (left) for whole-brain serial imaging. Inset: a coronal postimmunostained confocal image of the recorded (yellow) and local presynaptic cells (red) is shown.
(C) Left: membrane-voltage traces recorded at and two times the rheobase. Top left: the instantaneous frequency of AP firing at two times the rheobase is shown. Right: tuning polar plots of the same CC cell recorded during delivery of plasmids for RV targeting and tracing.
(D) Top: coronal two-photon whole-brain image stack showing the location of cells labeled with the modified rabies virus following electrophysiological characterization of the recorded cell in (C). Bottom: following imaging, the labeled cells were localized using a standard mouse brain atlas. Regions relevant to this study include the primary visual cortex (V1), the medial and lateral secondary visual cortices (V2M and V2L, respectively), the retrosplenial cortex (RSP), and the thalamus (TH).
(E) Example coronal images of the marked location of labeled cells (red spheres) within V1 (local) and outside V1 (long range).
(F) Histogram showing the relative distribution of labeled cells (n = 3 mice).
Error bars show SEM.