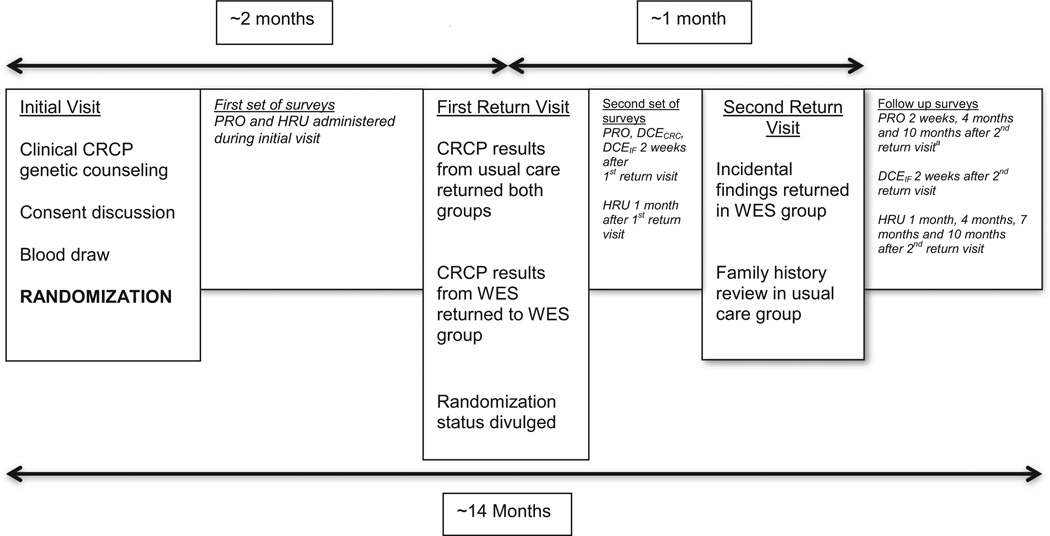
Fig. 1.
Study timeline. Patients are assessed at their initial clinic visit for inclusion/exclusion criteria, and if eligible, asked if they wish to enroll in the study and consented. Blood volumes are drawn simultaneously for usual care and the research protocol, followed by randomization to one of two groups: WES plus usual care or usual care alone. Results related to colorectal cancer/polyposis are returned in the first return visit, two months after the initial visit. During a second return visit that takes place one month after the first, incidental findings from WES are discussed in this group, while further review of family history takes place for the usual care alone participants. Patient reported outcomes (PRO), health resource utilization (HRU) and discrete choice experiment instruments (DCECRC for findings associated to colorectal cancer and polyposis and DCEIF for incidental findings) are given to the patient starting during the initial visit until 10 months after the second return visit. aThe PRO instrument sent 4 months after the second visit contains only the FACToR instrument.