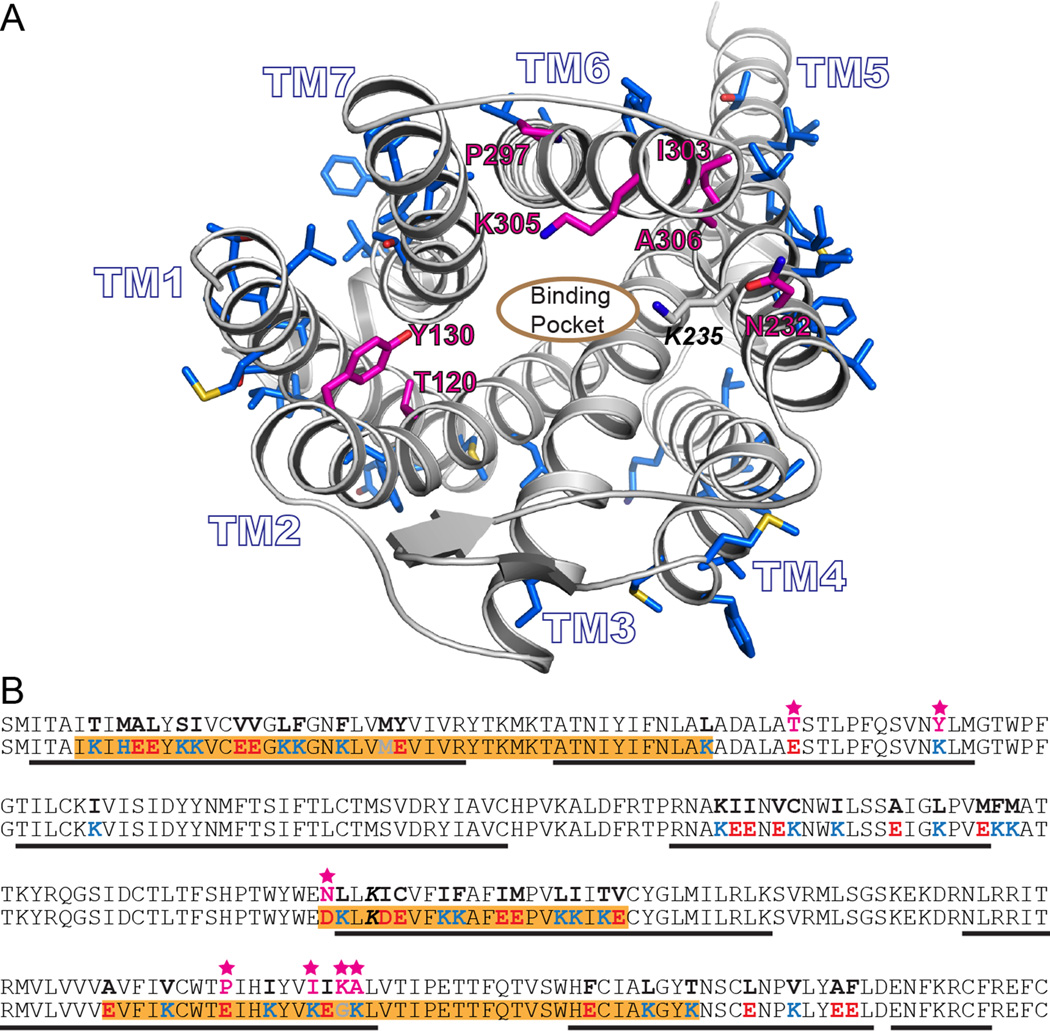
Figure 1.
A) Rendering of a model based on the murine mu opioid receptor crystal structure. The side chains of residues computationally designed to generate wsMUR-TM are rendered blue. The root-mean-square deviation of the Cα atoms between this previous comparative model model and the murine structure involving transmembrane domain residues is 2.60Å.8 The designed positions selected to be mutated back to the human native identities are shown in magenta (E120T2.54, K130Y2.64, D232N5.36, E297P6.50, K303I6.56, G305K6.58, and K306A6.59, see Materials and Methods for description about Ballesteros and Weinstein indexing.10 B) Sequences of the transmembrane domain of wild-type human μ opioid receptor (top) and the designed water-soluble variant wsMUR-TM (bottom) are displayed. Highlighted in orange are the segments of the designed sequence (wsMUR-TM) identified to be important to preserve protein expression. Seven residues that have been restored to native identities are starred and colored in magenta. wsMUR-TM = first variant of the water-soluble human μ opioid receptor transmembrane portion; wsMUR-TM_v2 = modified (second) variant of the water-soluble human μ opioid receptor transmembrane portion; TM = transmembrane; M/Z = mass-to-charge ratio.