Abstract
The mammalian reproductive hormone axis regulates gonadal steroid hormone levels and gonadal function essential for reproduction. The neuroendocrine control of the axis integrates signals from a wide array of inputs. The regulatory pathways important for mediating these inputs have been the subject of numerous studies. One class of proteins that have been shown to mediate metabolic and growth signals to the CNS includes Insulin and IGF-1. These proteins are structurally related and can exert endocrine and growth factor like action via related receptor tyrosine kinases. The role that insulin and IGF-1 play in controlling the hypothalamus and pituitary and their role in regulating puberty and nutritional control of reproduction has been studied extensively. This review summarizes the in vitro and in vivo models that have been used to study these neuroendocrine structures and the influence of these growth factors on neuroendocrine control of reproduction.
Keywords: GnRH, Kisspeptin, GPR54, insulin, IGF-1, pituitary, gonadotroph, GnRHR, Cre/LoxP, obesity, puberty
1. Introduction
The complexity of signaling to the hypothalamic-pituitary structures that control reproductive function is daunting. Mammalian reproduction is impacted by a wide range of environmental, social and proprioceptive cues. Information about season, time of day, proximity and sexual receptivity of a mate and nutritional availability all play an important role in modifying the activity of the hypothalamic-pituitary-gonadal hormone axis. In addition, signals from within the body such as infection, perceived stress, gonadal steroid hormone levels and energy availability can exert a profound impact on reproductive function (Whirledge and Cidlowski. 2010, Navarro and Kaiser. 2013, Legan and Karsch. 1975, Rivier. 1993, Bakker et al. 2001).
A number of laboratories have explored the regulation of the hypothalamic gonadotropin-releasing hormone (GnRH) neuron and the pituitary gonadotroph by the growth factors insulin and IGF-1, and have identified key roles for each in regulating reproductive development and function. The identification of a key role for kisspeptin (Kiss1) neurons in regulating GnRH neurons expands the repertoire of possible targets for growth factor influence on neuroendocrine reproductive function. Assembled here is a review of our current understanding of the role these structurally similar proteins play in regulating puberty, female cyclicity and the response of the hypothalamic pituitary (HP) axis to changes in metabolic status.
We summarize here the role of insulin and IGF-1, two proteins that can function as either hormones or growth factors in regulating key elements of the hypothalamus and pituitary that impact reproduction. Insulin and IGF1 evolved from a common hormone that regulated both growth and metabolic function and diverged during the development of vertebrates (LeRoith et al. 1993, McRory and Sherwood. 1997). The proteins are structurally homologous, bind to highly homologous receptors, and activate common signaling pathways. In addition, the source of both insulin and IGF-1 can be from the periphery (Hiney et al. 2009, Baura et al. 1993) or the brain (LeRoith et al. 1988, Daftary and Gore. 2003). These features of their structure, origin and function make it challenging to isolate the contributions of each hormone and the relative impact of peripheral levels to brain function. The use of in vitro and in vivo models, including novel genetic mouse models, has revealed fundamental clues to the roles of insulin and IGF-1 in normal development and physiology, and in pathophysiology, of reproductive function and we attempt to summarize those finding here.
2. Summary of HPG axis
The architecture of the HPG axis is classically comprised of three separate structures. GnRH neurons at the top of the axis direct the action of the pituitary and subsequently the gonads. GnRH neurons secrete the GnRH decapeptide into the fenestrated capillaries of the portal vasculature where it travels directly to the cells of the anterior pituitary. As is the hallmark of neuroendocrine regulation, the small volume of the portal circulation requires only picomolar amounts of GnRH for biological action. GnRH stimulates its receptors on the surface of the pituitary gonadotrophs to regulate the expression and secretion of the pituitary gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH). LH and FSH, in turn, regulate gamete maturation and the synthesis and secretion of the gonadal steroid hormones.
2.1 GnRH neurons
GnRH neurons lie scattered from the olfactory cortex to as far caudally as the median eminence in some mammalian species (King and Rubin. 1992). This scattered distribution reflects a dramatic migration from the site of origin of the GnRH neuron in the olfactory placode of the developing nose, across the nasal septum, through the olfactory bulbs and into the rostral hypothalamus (Schwanzel-Fukuda and Pfaff. 1989, Wray et al. 1989, Kim et al. 1999). They extend processes mainly to the organum vasculosum of the lamina terminalis (OVLT) and the median eminence (Jennes and Stumpf. 1986, King et al. 1982, Herde et al. 2011) both of which are circumventrictular organs located in the hypothalamus. Circumventricular organs are regions of the brain open to the peripheral circulation; the proximity of GnRH dendritic terminals to the OVLT allows for direct regulation by factors circulating in the blood such as metabolic or immune signals (Herde et al. 2011).
The GnRH neurons are particularly difficult to study due both to their low abundance in the brain (estimated as few as 800 neurons in the mouse (Wray et al. 1989)) and as few as 1200 GnRH neurons in human (Crowley. 2011), and to their diffuse and widely scattered distribution. Despite these obstacles the GnRH gene was cloned in a number of species (Bond et al. 1989, Hayflick et al. 1989, Radovick et al. 1990, Kepa et al. 1992), and has been found to be about 4Kb in length and to contain four exons. The first exon codes for a signal peptide and within the second exon is the coding sequence for the GnRH decapeptide. The remainder of the second exon, the third exon and most of the fourth exon code for the GnRH associated peptide (GAP) (Adelman et al. 1986, Dong et al. 1996, Mason et al. 1986) a cleavage product of unknown function that is produced during the processing of the GnRH prohormone. The promoters of the human, rat and mouse GnRH genes have been characterized and regions important for cell-specific expression and regulation of the gene have been identified (Kim et al. 2002, Wolfe et al. 2002, Wolfe et al. 1995, Skynner et al. 1999, Pape et al. 1999, Lawson et al. 1998, Whyte et al. 1995, Novaira et al. 2012, Novaira et al. 2011). The cell-specific regions of the human (Radovick et al. 1991) and rat (Mellon et al. 1990) GnRH promoters have been used to target large T antigen expression to GnRH neurons in transgenic mice that have been used to develop cell line models. These cell lines have proven to be important for understanding the electrophysiological, molecular and structural properties of GnRH neurons (Wolfe et al. 2002, Zhen et al. 1997, Anderson et al. 1999, Bosma. 1993, Besecke et al. 1994, Van Goor et al. 2000, Herbison and Moenter. 2011). A novel strategy for the development of a GnRH neuronal cell line was used by Salvi et al. to develop the Gnv-3 cells (Salvi et al. 2006). Dispersed adult hypothalamic cells were immortalized with a c-myc oncogene and the Gnv-3 clonal line obtained. These cells represent an adult cell model.
Mice expressing the CRE recombinase in GnRH neurons have been developed and have allowed for the production of a variety of conditional KO mouse models. These include the GnRH-CRE mouse, developed in our laboratory and the LHRH::CRE (Yoon et al. 2005) and GnRH-iCre mice (Shimshek et al. 2006) mice. The GnRH-CRE transgene contains 3446bp of the mouse GnRH promoter upstream of a CRE cDNA containing an artificial intron for improved expression in mammalian systems (Bunting et al. 1999). The LHRH::CRE mice were produced with a BAC transgene that includes 137 kb of 5′ upstream and 73 kb downstream sequences around the GnRH coding sequences. The GnRH-iCre mice were produced using a transgene consisting of 3.5kb of the mouse GnRH promoter fused to an improved CRE (iCRE) (Shimshek et al. 2002) designed to reduce the CpG content of the prokaryotic coding sequence with the intent of reducing epigenetic silencing in mammals. These models have been used for the targeted disruption of Otx2 (Diaczok et al. 2011), PI3 Kinase (Acosta-Martinez et al. 2009), ERK (Wierman et al. 2012), glutamate receptors (Shimshek et al. 2006), ERbeta (Abraham et al. 2003), GABA(A) receptor (Lee et al. 2010), leptin receptor (Quennell et al. 2009), Jak2 (Wu et al. 2011), insulin receptor (Divall et al. 2010), IGF-1 receptor (Divall et al. 2010) and recently the Kiss1 receptor (Kiss1R, also known as GPR54 (Novaira et al. 2013, Kirilov et al. 2013)) to the GnRH neurons.
2.2. Kisspeptin neurons and the kisspeptin receptor
The description of a patient with idiopathic hypogonadotropic hypogonadism with a mutation in the KISS1R, and a mouse model with a deletion of the Kiss1R gene added a new member to the axis structure (Seminara et al. 2003, de Roux et al. 2003). KISS1R is located on the short arm of chromosome 19 in humans and on chromosome 10 in the mouse and contains 5 exons, encoding a protein product of 398 amino acids in humans and 395 amino acids in mice. The natural ligand for KISS1R is the family of kisspeptins encoded by the Kiss1 gene, which in mice produces a 130 amino-acid protein that is processed to generate the biologically active 54 amino-acid amidated protein, Kisspeptin 54 (Kp54, also known as metastin). The carboxyterminal region of Kp54 is responsible for receptor binding and this region is the most conserved between species (Colledge. 2008). Carboxy-terminal peptides of 14, 13 or 10 amino-acids (Kp14, Kp13 and Kp10) show similar activities in vitro to Kp54 with the carboxy-terminal amidation required for proper function of all the kisspeptins (Ohtaki et al. 2001, Muir et al. 2001). The Kiss1 gene consists of two splice variants that produce the same protein product (Tomikawa et al. 2012).
Several studies have confirmed that GPR54 co-localizes with GnRH neurons (Han et al. 2005, Parhar et al. 2004, Irwig et al. 2004, Messager et al. 2005). Central or systemic administration of kisspeptins leads to GnRH and gonadotropin secretion in both prepubertal and adult animals (Irwig et al. 2004, Messager et al. 2005, Gottsch et al. 2006, Matsui et al. 2004, Plant and Barker-Gibb. 2004, Navarro et al. 2005, Shahab et al. 2005, Greives et al. 2007). Two principal populations of kisspeptin neurons are described in the hypothalamus, one in the arcuate nucleus and one in the anteroventral periventricular nucleus (AVPV). The specific subset of neurons in the AVPV have been shown to be critical intermediates in transducing estradiol positive feedback regulating GnRH and the subsequent LH surge that is required to induce ovulation (Popolow et al. 1981, Wiegand et al. 1980, Wiegand and Terasawa. 1982, Gu and Simerly. 1997). Within the AVPV of female mice, Kiss1-expressing neurons are activated by estradiol via the estrogen receptor alpha isoform (ESR1: (Wintermantel et al. 2006, Smith et al. 2005a)). In contrast, ARC Kiss1 expression is inhibited by estradiol (Smith et al. 2005a, Smith et al. 2005b), also mediated by ESR1, although a direct role in mediating negative feedback is not yet established.
In the last ten years kisspeptin neurons have been identified as significant players in the pubertal process (Seminara et al. 2003, Han et al. 2005, Shahab et al. 2005, Lomniczi et al. 2013). Lying afferent to GnRH neurons, the kisspeptin neurons have also been proposed to relay metabolic (Kalamatianos et al. 2008, Quennell et al. 2011, Wahab et al. 2011, Castellano et al. 2010a) or immune/inflammation status (Castellano et al. 2010b) to the GnRH neuron, to form the anatomical basis for negative and positive feedback by gonadal steroid hormones (as discussed above) and even to underlie the pulsatile secretion of GnRH (Okamura et al. 2013, Wakabayashi et al. 2013, Goodman et al. 2013). The kisspeptin neurons appear to serve as an integrating center upstream of the GnRH neuron.
The development of mice expressing CRE in the Kiss1 neurons have provided a valuable new tool for studying the neuronal regulation of reproduction. Three different lines have been developed. The KissIC mice were developed by the Boehm lab and were produced using a targeted insertion of a Kiss1 allele with the Cre cDNA inserted into exon 2 of the Kiss1 gene (Mayer and Boehm. 2011, Mayer et al. 2010). These mice have been used to suggest that puberty can proceed in the absence of Kiss1 suggesting functional redundancy in the systems controlling puberty (Mayer and Boehm. 2011). These mice have also been used to disrupt Esr1 in Kiss1 neurons (Mayer et al. 2010), and this study is discussed below. The Kiss1-Cre mice developed by the Elias laboratory used a BAC transgene to target expression to Kiss1 neurons (Cravo et al. 2011). These mice have been used to demonstrate that leptin regulation of reproductive function was not mediated by Kiss1 neurons (Donato et al. 2011) and to study the impact of insulin in kisspeptin neurons (discussed below and (Qiu et al. 2013)). These mice have also been used to disrupt Esr1 expression in Kiss1 neurons (Frazao et al. 2013). The Kiss1-CreGFP mice produced by the Steiner laboratory (Popa et al. 2013) used a knock-in strategy to insert a CRE-GFP fusion cassette upstream of the Kiss1 coding sequence. This insertion produced a hypomorphic allele that expressed very low levels of Kiss1 somewhat reducing their utility as a functional model for targeted gene ablation. However, these mice have been used to identify Kiss1 neurons in slice preparation and to explore the electrophysiological properties of arcuate Kiss1 neurons (Gottsch et al. 2011).
2.3. Pituitary gonadotroph
In the gonadotroph, GnRH binds to specific membrane receptors (GnRHR) on the cell surface. Activation of GnRHRs triggers a cascade of intracellular signaling pathways, which ultimately lead to the synthesis and release of LH and FSH. LH and FSH are glycoprotein hormones that belong to a family that also includes thyroid-stimulating hormone (TSH) and chorionic gonadotropin (CG). Members of the glycoprotein hormone family are heterodimers consisting of two non-identical and non-covalently linked subunits. The α subunit (glycoprotein hormone alpha, αGSU) is common to all members of this family and is expressed in molar excess in the pituitary to facilitate formation of the intact hormone. The β subunit is unique and confers specific biological activity to each dimeric hormone (Pierce and Parsons. 1981). Expression of genes for LHβ (Lhb), FSHβ (Fshb), and αGSU (Cga) in the anterior pituitary is dependent on pulsatile GnRH secretion. The pattern of the pulse determines the differential expression of the transcripts. Rapid, high-amplitude pulses stimulate an increase in Lhb mRNA levels, leading to an increase in LH synthesis and release from the gonadotroph (Haisenleder et al. 1991, Ferris and Shupnik. 2006).
Several mouse gonadotroph cell lines have been developed by the Mellon laboratory and are thought to represent different stages of pituitary development. The αT1-1 (Alarid et al. 1996) cell line was developed from a mouse containing a transgene with 5.5kb of the human common glycoprotein alpha (CGA) gene promoter upstream of the T antigen oncogene. The αT1-1 is thought to be a cell line representing an early pituitary lineage. These cells express the Cga gene but do not express the GnRH receptor or glycoprotein beta subunit genes. The αT3-1 cells (Windle et al. 1990) were obtained from mice containing a transgene in which 1.8kb of the human CGA gene promoter drove expression of the T antigen oncogene. These cells represent a later developmental stage of pituitary development and express the GnRH receptor (Tsutsumi et al. 1992) and respond to GnRH (Windle et al. 1990). Several other cell lines have been obtained from naturally occurring human or rat pituitary tumors (reviewed in (Ooi et al. 2004)) and in transformed adult pituitary cells (Kim et al. 2011), but only the LβT2 cells, also produced by the Mellon group, have been shown to express Fshb. The LβT2 gonadotroph cell line (Windle et al. 1990) was obtained from a mouse with oncogene targeting using 1.2kb of the human LHB subunit gene promoter. Originally reported to produce LH and GnRHR but not FSH (Alarid et al. 1996) they were subsequently found to secrete FSH, but only following stimulation by activin and not in response to GnRH treatment (Graham et al. 1999). These cells have become the go to model system for understanding regulation of pituitary function.
Pituitary gonadotroph specific CRE expressing mice have been developed by several groups. Two different laboratories have produced mice expressing Cre under the control of about 4.5kb of the murine promoter for the Cga gene (Cushman et al. 2000, Singh et al. 2009), and these mice have been used to delete Esr1 from pituitary gonadotrophs (Singh et al. 2009, Gieske et al. 2008). These mice have also been used to reveal a role for ERK signaling in the gonadotroph (Bliss et al. 2009) and for insulin receptor mediated signaling in the development of obesity induced infertility ((Brothers et al. 2010) and discussed below).
Other models have been developed including the Tg(Lhb-cre)1Sac mice (Charles et al. 2008) and the GnRHR cre mouse. (Wen et al. 2008). These mice have been used to label the gonadotropes to study their phenotypic variability (Wen et al. 2008), to knock out the FoxL2 gene (Tran et al. 2013) and to explore the timing of the developmental role for PTX2 in the pituitary (Charles et al. 2008).
2.4. Gonads
Following GnRH stimulation of the pituitary gonadotroph, LH and FSH are released into the general circulation and bind to specific receptors in the gonads. LH binds to LH receptors in the theca cells of the ovary and the Leydig cells of the teste. In both, it stimulates expression of enzymatic machinery required for androgen synthesis (Hedin et al. 1987, Hedin et al. 1987, Payne. 1990, Bogovich and Richards. 1982, Ma et al. 2004). Testosterone produced by the Leydig cell diffuses into the seminiferous tubules and androgen action at the level of the Sertoli cell is essential for sperm production in males (De Gendt et al. 2004). In the ovary, androgen (predominantly androstenedione) from the theca cell serves as a substrate for estradiol synthesis by the granulosa cells. Conversion of androgens to estradiol is catalyzed by Cyp19 (aromatase) which is regulated by FSH action in the granulosa cells (Hillier et al. 1981, Ryan. 1979). The massive gonadotropin surge that triggers ovulation is followed by the formation of the corpus luteum from the cells of the ruptured follicle. The development of the corpus luteum coincides with the granulosa cells of the ovary beginning to express the LH receptor. Receptor activation by LH or chorionic gonadotroph, produced by the syncytiotrophoblast, and later the placenta, drives synthesis and secretion of progesterone required for the maintenance of pregnancy (reviewed in (Stocco et al. 2007)).
3. Puberty
The onset of reproductive function, puberty, is activated by an awakening of the GnRH pulse generator that is dormant during adolescence. In humans GnRH secretion during adolescence is restrained by an unknown mechanism that does not appear to involve gonadal steroid hormone feedback since patients with gonadal dysgenesis exhibit a rise in pituitary gonadotroph secretion when puberty normally occurs (Ross et al. 1983). Similar findings were observed in the neonatally gonadectomized rhesus monkey model model (Suter et al. 1999, Pohl et al. 1995, Terasawa et al. 1984). In other animal models including sheep and rodent, a gonadostat model has been proposed in which the gonadal steroid hormones exert a negative feedback on pulse generator activity during adolescence that appears to be released at puberty to drive an increase in gonadotropin secretion (Andrews et al. 1981, Olster and Foster. 1986). Strong support for this model comes from a recent study in which Esr1 was deleted from the Kiss1 neurons resulting in a dramatic advance in the age of puberty in female mice (Mayer et al. 2010). However, these mice also exhibit impaired reproductive cyclicity and are infertile confirming an important role for estradiol feedback regulation at the level of the Kiss1 neuron. A subsequent study knocking out Esr1 in Kiss1 neurons, using the Kiss1-Cre mouse, observed a similar phenotype (Frazao et al. 2013). Whether steroid dependent or not, the factors contributing to the initiation of pulsatile secretion of GnRH at puberty are the subject of intense study. For over 30 years circulating IGF-1 has been implicated as a circulating factor contributing to the timing of puberty (Copeland et al. 1982, Luna et al. 1983, Hiney et al. 1991, Hiney et al. 1996). A recent study has also provided evidence that insulin may also contribute to the progression of puberty (Qiu et al. 2013). The role of IGF-1 and insulin in the regulation of neuroendocrine function controlling reproduction is reviewed below.
In addition to its potential role in mediating positive steroid hormone feedback at the time of the LH surge, kisspeptin may play an important role in mediating pubertal onset. Both Kiss1 mRNA and Kiss1R mRNA increase during the pubertal development process in rats and monkeys (Muir et al. 2001, Keen et al. 2008, Kotani et al. 2001) and Kiss1 mRNA increases dramatically in mice during this period of time (Clarkson and Herbison. 2006). In addition, centrally administered kisspeptin stimulates gonadotropin secretion and induces pubertal development in juvenile rodents and monkeys (Matsui et al. 2004, Shahab et al. 2005, Navarro et al. 2004).
4. Description of metabolic regulation of reproduction
An important evolutionary adaptation is the tight coupling of puberty and reproductive function and behavior to nutritional availability and to the nutritional status of the organism. Both chronic and short-term withdrawal of nutrients has been shown to inhibit reproduction in mammals. Even four hours after missing a meal, the pulse frequency of LH release in Rhesus monkeys is dramatically reduced (Schreihofer et al. 1993b, Helmreich and Cameron. 1992). This effect appears to be due to metabolic changes and not psychological stresses (Schreihofer et al. 1993b, Schreihofer et al. 1993a). In humans, functional hypothalamic amenorrhea (FHA) represents a common cause of infertility in women and is often the result of a metabolic stress from low weight or excessive exercise (Berga et al. 1989, Laughlin et al. 1998). A psychogenic component is also often a basis for FHA (Berga et al. 1997, Giles and Berga. 1993) which means that even mild metabolic stresses can impact reproductive function when coupled with more extreme psychosocial stress.
Recent studies from the Bathea lab promise to reveal the neurobiological underpinning to FHA. Female cynomolgus monkeys were screened for their response to combined mild metabolic and mild psychosocial stress. Monkeys were stratified into stress resistant, moderately stress resistant and stress sensitive based on the response of their reproductive hormone axis to the experimental paradigm. Monkeys defined as stress sensitive were those that responded with a rapid cessation of reproductive function and serve as a model of FHA (Senashova et al. 2012). Stress sensitive monkeys were found to have increased cellular content of GnRH coupled with reduced GnRH fiber density at the median eminence (Centeno et al. 2007b), reflecting reduced secretory activity (Herod et al. 2011b). Using this model allowed investigators to identify a key role for impaired serotonin function (Lima et al. 2009, Centeno et al. 2007a) which, in turn, produces changes in the neural circuitries controlling the hypothalamic-pituitary-adrenal stress axis (Herod et al. 2011a, Bethea et al. 2011).
It is unclear whether metabolic or secondary hormonal signals modify the central signal regulating puberty and reproduction in humans, monkeys or other mammals. A number of candidate molecules have been proposed including glucose (Oltmanns et al. 2001, Rodriguez et al. 1999, Kawaguchi et al. 1998, He et al. 1999), fatty acids (Boukhliq and Martin. 1997, Schneider and Wade. 1989, Schneider and Wade. 1990), insulin (Bruning et al. 2000, Dong et al. 1991, Tanaka et al. 2000), growth hormone/IGF-1 (Hiney et al. 2009, Anderson et al. 1999, Hiney et al. 1991, Hiney et al. 1996, Longo et al. 1998), leptin (Ahima et al. 1996, Ahima et al. 1997, Mounzih et al. 1997, Cheung et al. 1997, Chehab et al. 1997) and a number of other adipokines (Mitchell et al. 2005, Lu et al. 2008) and gut peptides (Giacobini and Wray. 2007, Fernandez-Fernandez et al. 2005, Tena-Sempere. 2008). While it is well known that insulin levels are low during fasting, peripheral IGF-1 levels also change dramatically during fasting in humans (Clemmons et al. 1981, Stoving et al. 1999) and rodents (Powolny et al. 2008, Frystyk et al. 1999) although the drop in insulin is more acute than the reduction in IGF-1. Powolny et al. also observed a dramatic increase in IGFBP3 suggesting an even more robust decrease in bioavailable IGF-1 in response to nutritional deprivation (Powolny et al. 2008). It is unknown if production of insulin or IGF-1 by the brain is similarly blunted by fasting. Insulin levels are elevated in states of obesity (reviewed in (Guilherme et al. 2008)). The initial increase in insulin levels is thought to compensate for peripheral insulin resistance caused by obesity. Elevated insulin levels are reduced by weight loss and insulin sensitizing drugs such as metformin. There are conflicting reports regarding IGF-1 levels in obesity. Overfeeding normal weight individuals results in an increase in IGF-1 levels (Forbes et al. 1989). However, in obese adults, levels of IGF-1 are usually found to be normal despite low growth hormone levels (Nam et al. 1997, Woelfle et al. 2007) although some have reported that free IGF-1 levels are elevated in non-diabetic obese adults when compared to lean controls (Frystyk et al. 1999). Therefore these signals may represent good candidates for mediating signals about nutritional status.
Studies designed to determine metabolic factors that regulate the reproductive hormone axis have been performed in myriad animal models; differences in the outcomes may result from differences in experimental design, from an incomplete understanding of the mechanisms involved or from different species using different modes of regulation. Additional complexity arises because metabolic cues that regulate GnRH neuronal activity can be exerting their effects directly at the level of the GnRH neuron, or indirectly by regulating any number of neuron groups that have been proposed to be afferent to GnRH neurons. For example, there is extensive evidence that leptin regulates reproductive function, however leptin receptors do not appear to be located on GnRH neurons (Quennell et al. 2009) and leptin appears to exert its effects on the GnRH neurons indirectly (Korner et al. 2001, Leslie et al. 2001, Vulliemoz et al. 2005, Lebrethon et al. 2000, Parent et al. 2000). Smith et al. proposed that leptin’s effect may be mediated by Kiss1 neurons (Smith et al. 2006), but recent work from the Elias lab cast doubt on that neuronal circuit (Donato et al. 2011). However, kisspeptin expression is certainly decreased following fasting (Castellano et al. 2005), and has been proposed to be an important afferent input to the GnRH neuron in the regulation of metabolic signals which will be discussed below.
An interesting paradox is that at the opposite end of the metabolic spectrum there is also a decrease in reproductive function. Obesity is associated with reproductive dysfunction in adults and children. Obese, oligomenorrheic women with or without polycystic ovarian syndrome have increased LH pulsatility compared to controls (Apter et al. 1994), thought to be mediated by an increase in GnRH pulsatility (Hayes et al. 1998, Kazer et al. 1987). Female mice with diet induced obesity (DIO) have been shown to develop reproductive dysfunction compared to lean controls (Brothers et al. 2010, Tortoriello et al. 2007, Tortoriello et al. 2004). These mice also exhibited hyperinsulinemia and hyperleptinemia. The complex biochemical profile of obese rodents has made it challenging to dissect the specific factors that contribute to their hypothalamic reproductive dysfunction. Studies from our laboratory suggest that insulin signaling may mediate some of the effects of obesity that contribute to infertility (Brothers et al. 2010, Wu et al. 2012) and will be discussed in more detail below.
5. Insulin and IGF-1 share homology in structure and have overlap in receptor activation and in their signaling pathways
Insulin and IGF1 and 2 are members of the same family of insulin-like peptides. The three dimensional structure of insulin and the IGF peptides is similar (Cooke et al. 1991), but there are significant differences in the binding affinity of insulin, IGF1 and IGF2 to their respective receptors. The insulin and IGF-1 receptors (IR and IGF1R, respectively) exist as a heterotetramer composed of two α/β dimers (Nakae et al. 2001) although hybrids consisting of combinations of IR and IGF1R subunits have been reported (Benyoucef et al. 2007, Soos and Siddle. 1989, Treadway et al. 1989, Moxham et al. 1989). Insulin and IGF-1 have low nM affinity for their cognate receptors (~1–5nM), but bind to their non-cognate receptor with an order of magnitude lower affinity (Kjeldsen et al. 1991, Gauguin et al. 2008) and to hybrid receptors with varying affinities (Benyoucef et al. 2007, Sakai and Clemmons. 2003) suggesting that cross-talk of signaling for insulin and IGF-1 could take place at the receptor level.
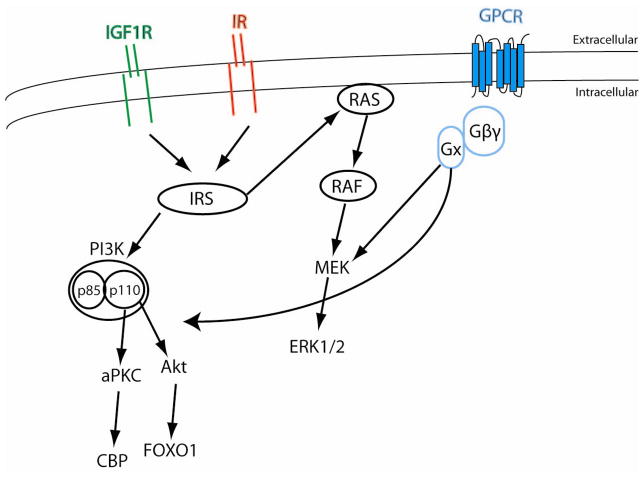
Upon binding to ligand, the IR/IGF-1R undergoes conformational changes that result in autophosphorylation of the cytoplasmic portion of the β subunit. A number of downstream signaling pathways are used by both IR and IGF-1Rs (schematized in Figure 1) indicating that specificity is potentially regulated by anatomical distribution of ligand and receptor. Phosphorylation of the tyrosine subunits of the β subunit of the receptor can directly phosphorylate the insulin receptor substrates (IRS1-4; reviewed in (Giovannone et al. 2000)) which in turn phosphorylates p85, the regulatory subunit of phosphoinositide 3-kinase (PI3K), leading to an activation of p110, the catalytic subunit of the PI3K. Activation of the PI3K pathway stimulates Akt activity and atypical protein kinase C activity (Egawa et al. 2002). This pathway is generally important for glucose transport and glycogen synthesis (Czech and Corvera. 1999, Bandyopadhyay et al. 1997). This branch of the insulin signaling tree has been shown to regulate the FoxO1and CBP transcription factors (Nakae et al. 2002, Zhou et al. 2004, Zanger et al. 2001) which, as discussed later, may represent nodes of intersection for GnRH and insulin/IGF-1 action in the pituitary gonadotroph. IR and IGF-1R signaling can also be meditated by a Ras/Raf/Mek pathway which is broadly involved in the regulation of proliferation, differentiation and apoptosis (Coolican et al. 1997, Seger and Krebs. 1995). The Ras signaling cascade can result in stimulation of ERK1/2 (also referred to as p42/p44 MAP kinase) and also the Jun N-terminal Kinases (JNK, also referred to as Stress Activated Protein Kinases, SAPK). The MAP kinase JNK is thought to contribute to activation of the AP1 complex by activating Jun (Davis. 2000). Interestingly JNK-1 plays a role in mediating insulin resistance in obesity perhaps by catalyzing inhibitory serine phosphorylation of IRS molecules in the liver (Hirosumi et al. 2002, Yang et al. 2007). The overlap between the signaling pathways for insulin and IGF-1 indicate that the temporal and spatial expression of the receptors underlies the specificity of the responses.
Figure 1. Common signaling pathways activated by insulin and IGF-1 receptors.
Both insulin and IGF-1 receptors are receptor tyrosine kinases. Receptor activation results in autophosphorylation of receptor which in turn can activate IRS1-4. IRS can activate the Ras GTPase. Ras induces sequential activation of Raf, MEK and ERK1/2. Activated IRS protein can also activate the regulatory subunit of the PI3-kinase, p85, which in turn stimulates the p110 catalytic subunit of PI3-Kinase. PI3-Kinase is a lipid kinase that induces an inositol phosphate cascade resulting in activation of Akt and the atypical PKC (aPKC). G-protein coupled receptor (GPCR) is also indicated representing kiss1 receptor in the GnRH neuron or the GnRH receptor in the pituitary gonadotroph. Binding by ligand induces conformation changes that result in activation of an associated G-protein by exchanging its bound GDP for a GTP. The α subunit of the G-protein complex (Gαx = Gαs, Gαi, Gαq) dissociates from the β and γ subunits to further affect intracellular signaling proteins (Gαs activates adenylyl cyclase, Gαi, inhibits adenylyl cylcase, Gαq activates phospholipase C. Both kiss1 and GnRH can activate canonical insulin/IGF-1 signaling pathways. CBP (CREB binding protiens) and FOXO1 represent potential intersecting nodes for insulin/IGF-1 signaling and GnRH signaling in the pituitary gonadotroph.
6. Insulin regulation of neuroendocrine function
6.1 Insulin regulation of hypothalamic GnRH and kisspeptin neurons
There is evidence that insulin may play a role in the regulation of puberty and reproductive function. Polycystic ovary syndrome (PCOS) is a human clinical condition marked by amenorrhea and hyperandrogenism. PCOS patients frequently have high testosterone levels and insulin resistance associated with high insulin levels. The majority of cases also have an accelerated GnRH pulsatility that produces high LH levels (Hayes et al. 1998, Kazer et al. 1987). In contrast, a reduction of GnRH pulse generator activity has been reported in women with functional hypothalamic amenorrhea (FHA) that generally have reduced insulin levels (Laughlin et al. 1998) compared to women with regular menses. Epidemiological evidence strongly suggests that increased insulin resistance and hyperinsulinemia, with or without obesity, contributes to an advance in the age of pubertal onset in boys and girls (Wilson and Umpierrez. 2008, Slyper. 2006, Ibanez et al. 1997). In contrast, patients with type 1 diabetes have been reported to have a delay in puberty (Elamin et al. 2006) although this is not uniformly observed (Salerno et al. 1997) and difference in the progression of puberty in this population may be secondary to decreased weight.
Animal and tissue culture models have been used to explore the effects of insulin on the reproductive hormone axis. A number of groups have reported that reduced insulin levels will result in attenuated GnRH pulsatility and axis activity in vivo (Dong et al. 1991, Tanaka et al. 2000, Castellano et al. 2006) and conversely, that increased insulin levels will stimulate GnRH neurons in vivo (Miller et al. 1995, Miller et al. 1998, Kovacs et al. 2002) and in vitro (Kim et al. 2005, Gamba and Pralong. 2006, Olson et al. 1995). Insulin receptors have been reported in the hypothalamus, at high levels in the arcuate nuclei, the SCN, and the para- and periventricular nuclei, but also at lower levels in the median eminence (Corp et al. 1986, Havrankova et al. 1978, van Houten et al. 1980). Although some systemic insulin likely enters the brain through the circumventricular organs, insulin can cross the blood brain barrier (Baskin et al. 1983).
The most compelling evidence for a role for insulin in the regulation of reproductive function comes from the production of a neuron specific insulin receptor knock out (NIRKO) mouse (Bruning et al. 2000). These mice were obese with elevated insulin levels, and sub-fertility as a result of impaired spermatogenesis and follicular maturation. The sub-fertility in these mice was due to a reduction in GnRH release and a consequent reduction in pituitary gonadotropin release. This study did not allow for the determination as to whether the insulin sensing neurons are the GnRH neurons themselves, or an upstream signaling neuron, but they do implicate signaling through insulin receptors in the brain as a mechanism for the regulation of reproductive function.
While GnRH neurons in the brain have not yet been demonstrated to express the insulin receptor, GnRH neuronal cell lines such as the murine GN11 and GT1-7 lines and the rat GNV3 lines have all been shown to express the insulin receptor (Salvi et al. 2006, Kim et al. 2005, DiVall et al. 2007). Using these cell lines, insulin has been shown to activate the ERK and PI3K signaling pathways (Salvi et al. 2006, Kim et al. 2005, DiVall et al. 2007) and to stimulate secretion of GnRH (Salvi et al. 2006). These data suggest that insulin action in the brain regulating the expression and secretion of GnRH could be exerted at the level of the GnRH neuron. Because these cell lines were derived from different developmental stages, insulin could play a role during the development of the GnRH neuron and an ongoing role in adult physiology.
In vitro evidence for a role for insulin directly at the level of the GnRH neuron has not yet been corroborated with in vivo studies. Both male and female mice with the insulin receptor disrupted in GnRH neurons were found to exhibit normal puberty and normal fertility (Divall et al. 2010) indicating that insulin action at this level does not affect GnRH neuron function in normal nutritional and physiological conditions. Since the deletion of insulin receptor occurred early in development, perhaps redundancy in regulatory pathways allowed normal reproductive function. Additionally, a phenotype in these mice may emerge under physiological or pathophysiological conditions that have not yet been experimentally explored. This scenario is described below for mice lacking the insulin receptor in the pituitary gonadotroph in which no phenotype was observed in mice fed a normal chow diet, but with a role for insulin being revealed in mice fed a high fat diet. These differences between the results obtained from cell line models and the mouse model underscore the need to validate data obtained in vitro with in vivo models.
Studies performed by Schneider and Wade have suggested that it is not activation of insulin receptors in the hypothalamus that regulates reproductive function, but rather the change in energy partitioning that results from insulin treatment (reviewed in (Schneider and Wade. 1989, Schneider and Wade. 1990, Wade and Jones. 2004)). These and other studies have identified the hindbrain as an important glucose sensing region regulating reproduction (Cates and O’Byrne. 2000), however, analysis of a mouse with a functional KO of the KATP channel has indicated that glucose sensing by the brain stem and hindbrain may not contribute to the fasting inhibition of reproductive function (Huang et al. 2008) as previously proposed (Cates and O’Byrne. 2000, Murahashi et al. 1996). The GnRH neuron itself could serve as a glucose sensor (Zhang et al. 2007, Roland and Moenter. 2011b) which could be mediated by the energy sensing signaling molecule, AMPK (Roland and Moenter. 2011a). However, data from in vivo promoter mapping studies from our laboratory suggest a direct role for insulin. Transgenic promoter reporter mice (Kim et al. 2002, Kim et al. 2007) were injected with insulin and hypothalamic luciferase levels measured by luminometer (Kim et al. 2002, Wolfe et al. 2002). This study was analogous to promoter mapping performed to identify a kisspeptin response element in the mouse GnRH promoter (Novaira et al. 2012). We found that insulin stimulated GnRH promoter activity and that an insulin responsive region of the mGnRH promoter lay between −2046 and −356bp upstream of the transcriptional start site (Figure 2) which corresponds to promoter elements mediating insulin action mapped in vitro (Kim et al. 2005). The study in mice was performed in conditions of insulin induced hypoglycemia, but also when blood glucose was restored to fed levels by IP injection of glucose. These data suggest that circulating levels of glucose are not primarily regulating GnRH neuron function at least at the level of GnRH expression. Our studies using the reporter mouse model, therefore, suggest that insulin signaling may play a role in GnRH neuron function in nutritionally extreme states.
Figure 2. Insulin increases GnRH promoter activity in vivo.
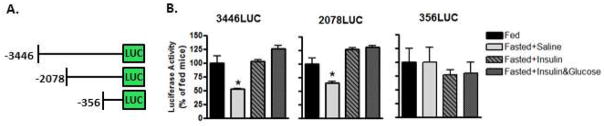
A. Transgenic mice bearing sequential deletions of the mGnRH promoter fused to the luciferase (LUC) reporter gene were used in the experiments. B. Hypothalamic luciferase activity (normalized to activity in fed mice) of transgenic male mice bearing the −3446LUC transgene, −2078 transgene, or the −356 transgene. Hypothalami were obtained one hour after intraperitoneal vehicle (saline), insulin (1mU/gm), and/or glucose (2 mg/gm). At the time, glucose levels in fasted mice that received saline or insulin were normal or low (95 ± 6 and 47±7 mg/dL, respectively) while glucose was at a similar level in fed mice and in mice administered insulin plus glucose (187±35 and 175± 48 mg/dL, respectively). * indicates a significant difference from fed (p<0.05).
Kisspeptin neurons have been proposed to play an important role in communicating information about metabolic status to the GnRH neurons (Castellano et al. 2010a, Castellano et al. 2005). Changes in insulin levels in response to metabolic status could be relayed by kisspeptin neurons. To study this as a potential mechanism for insulin regulation of GnRH neuronal function, Jennifer Hill’s group first demonstrated that kisspeptin neurons expressed the insulin receptor. They subsequently produced a kisspeptin-specific IR knock out mouse (Qiu et al. 2013) and documented a significant delay in puberty in both males and females, but no overt metabolic or reproductive phenotype in adults. The response of these mice to metabolic perturbations such as prolonged nutritional deprivation or diet induced obesity has not yet been explored. A kisspeptinergic neuronal cell line has not yet been developed rendering impossible the types of studies performed with the GT1-7 and GN11 GnRH neuronal cell lines exploring the mechanism of action of insulin or other modifying factors.
6.2. Insulin regulation of the pituitary gonadotroph
The LβT2 cells express the insulin receptor and have been used to explore insulin action in the gonadotroph (Buggs et al. 2006, Dorn et al. 2004, Navratil et al. 2009). Additionally, the pituitary gonadotroph expresses insulin receptors (Gutierrez et al. 2007, Soldani et al. 1994). While most of the studies using the LβT2 model system have suggested an augmentation of Lhb expression there are some differences obtained by different groups that should be noted. Our laboratory found that LβT2 cells respond to an insulin stimulus by increasing the effects of GnRH on LHβ mRNA levels (Buggs et al. 2006). This effect of insulin is debated as Dorn et al. found that while insulin was able to stimulate LHβ expression, it had no effect on GnRH stimulated Lhb expression in LβT2 cells (Dorn et al. 2004). Navratil et al. also observed an increase in Lhb synthesis in LβT2 cells, but proposed that insulin exerted its action by augmenting GnRH stimulated translation of the Lhb mRNA (Navratil et al. 2009).
Studies in humans have been equally inconclusive with some finding that insulin stimulated LH secretion in women (Moret et al. 2009) and some suggesting that insulin reduced baseline LH levels in women with or without PCOS (Lawson et al. 2008). These studies are difficult to interpret because the locus of action of insulin could be in the brain or at the level of the pituitary.
To understand the role of insulin in the pituitary gonadotroph in the normal development and function of the pituitary, we developed gonadotroph-specific insulin receptor KO mice (PitIRKO). Extensive reproductive phenotyping of females suggested that the PitIRKO mice exhibited a normal pubertal progression, normal estrous cyclicity, normal LH and FSH levels and, most importantly, normal fertility (Brothers et al. 2010). Additionally, no phenotype was observed in male PitIRKO mice (data not shown) which supports findings from human studies in male subjects that suggest no effect of insulin on reproductive neuroendocrine function (Pesant et al. 2012).
The lack of a phenotype in the female PitIRKO mice was not necessarily inconsistent with in vitro findings since comparisons of dose, timing and developmental stage between cell line models and in vivo are difficult. We proposed that insulin may still exert action in female mice, but only at high circulating levels. To test this hypothesis, we explored whether a phenotype might emerge in diet-induced obese (DIO) mice that exhibited compensatory hyperinsulinemia. These studies confirmed that there was a deleterious role for insulin signaling in the pituitary gonadotroph with insulin mediating a chronic increase in LH secretion that resulted in impaired estrous cyclicity and infertility (Brothers et al. 2010). These studies established a pathophysiological role for insulin in the development of obesity associated infertility Subsequent studies demonstrated that in mice fed a normal diet, the pituitary exhibited a lower sensitivity to insulin than classic metabolic tissues such as the liver and muscle. However, in contrast to the metabolic tissues that exhibited insulin resistance in the obese state, the pituitary did not exhibit a resistance to insulin in obese female mice (Wu et al. 2012). This study suggested a model in which it is not insulin resistance, but retained insulin sensitivity in a setting of high insulin levels, that produces infertility.
The mechanism of action of insulin in the gonadotroph is not well established. There are a number of potential nodes of intersection between GnRH receptor and insulin receptor mediated signaling which in large part reflects the diversity of signaling pathways activated by the GnRH receptor (Millar et al. 2004). The wide range of intracellular signaling pathways activated by the GnRH receptor is likely due to the receptor only having a cytoplasmic tail of two amino acids, a feature that makes the GnRHR unique among the class of GPCRs (Millar et al. 2004, Rispoli and Nett. 2005), and may be necessary to regulate an especially large number of separate processes including LH and FSH synthesis and secretion (Stanislaus et al. 1998a), GnRH receptor expression and GnRH receptor desensitization. GnRHR can couple with Gαq/11, GαI, and Gαs (Hawes et al. 1993, Stanislaus et al. 1998b), and has been shown to exert effects through known insulin targets such as ERK (Bliss et al. 2009), PI3K (Navratil et al. 2009), FOXO1 (Arriola et al. 2012) and CBP (Miller et al. 2012).
7. IGF-1 regulation of neuroendocrine function
7.1. IGF-1 regulation of hypothalamic GnRH and kisspeptin neurons
Data obtained from IGF-1 and IGF-1R KO mice has not elucidated a role for IGF-1 in the central reproductive axis. Although the IGF-1 KO mouse is infertile (Baker et al. 1993, Baker et al. 1996), the developmental defect was thought to be at the gonadal level. However, any alteration in GnRH or LH release was not examined. The IGF-1R KO mouse dies at birth due to respiratory failure (Liu et al. 1993) rendering an analysis of reproductive function impossible.
Changes in bioavailable IGF-1 in response to changes in the expression of the IGF binding proteins, however, can exert important effects on reproductive neuroendocrine function. IGF-1 is carried in the blood as part of a complex in association with an IGF binding protein (IGFBP) and an acid labile protein (Baxter et al. 1989). More than 95% of IGF-1 in the blood is bound to one of six IGF binding proteins (IGFBP 1–6) (Martin and Baxter. 1992, Jones and Clemmons. 1995). The IGFBPs have little affinity for insulin (Jones and Clemmons. 1995). The most abundant of these binding proteins is IGFBP3. Transgenic IGFBP3 over expressing female mice have slightly reduced litter sizes, although a neuroendocrine component to this reproductive phenotype was not discussed (Modric et al. 2001). IGFBP6 over expressing transgenic mice exhibit reduced LH levels to 50% of WT litter mates (Bienvenu et al. 2004). IGFBP1 over expressing transgenic females appear to exhibit a pituitary gonadotroph hyperplasia (Froment et al. 2002) while the IGFBP1 over expressing transgenic males secrete normal levels of LH in the face of three-fold reduced circulating testosterone levels suggesting neuroendocrine abnormalities exist in both sexes (Froment et al. 2004). IGFBP5 over expressing female transgenic mice also exhibit sub-fertility, although this was not confirmed to have a neuroendocrine component (Salih et al. 2004). More recent studies exploring the phenotype of mice with deletions in the IGFBP genes have not noted an effect on reproductive function (Ning et al. 2006) despite impaired growth and metabolic function. Given the complexity and range of proposed roles of the IGFBP- carrier proteins, cell-specific targeting molecules, ligands for cell surface receptors- much work is needed to clarify the contributions of these proteins to reproductive development and function.
IGF-1 has been demonstrated to affect GnRH neuronal function in vitro (Zhen et al. 1997, Anderson et al. 1999, Longo et al. 1998). IGF-1 stimulated GnRH release from GT1-7 cells (Anderson et al. 1999) and stimulates GnRH promoter activity in vitro (Zhen et al. 1997, Longo et al. 1998). An AP-1 site in the human GnRH promoter has been demonstrated to mediate IGF-1 stimulation of hGnRH promoter activity in vitro (Zhen et al. 1997). A classic AP-1 site is not found in the mouse promoter, leaving open the possibility that a different signaling mechanism may mediate IGF-1 stimulation of the promoter in the mouse. Fos/Jun heterodimers or Jun/Jun homodimers constitute the AP-1 transcription factor which binds to the consensus DNA sequence TGATCTA and can transactivate gene expression. Additional protein factors can form dimers with c-Jun to produce an AP-1 transcription factor; however c-Fos can only form stable heterodimers with Jun proteins (Shaulian and Karin. 2002). Since IGF-1 stimulates human GnRH promoter activity in the NLT mouse cell line and IGF-1 stimulates endogenous mouse GnRH gene expression in the same NLT cells (Zhen et al. 1997, Longo et al. 1998, Olson et al. 1995) it is possible that while there is divergence of the cis-regulatory elements between the mouse and human GnRH gene promoters there is conservation of the signaling pathways mediating IGF-1 regulation in human and mouse GnRH neurons. A similar phenomenon has been observed in the study of cell-specific expression of the GnRH gene. The promoter regions of the human GnRH promoter that target gene expression to GnRH neurons in transgenic mice have little homology with regions in the mouse or rat GnRH promoters (Hayflick et al. 1989, Kepa et al. 1992, Mason et al. 1986, Wolfe et al. 2002, Wolfe et al. 1995, Whyte et al. 1995, Kepa et al. 1996).
IGF-1Rs are located throughout the brain, including the hypothalamus (D’Ercole et al. 1996) and specifically are found on GnRH neurons (Daftary and Gore. 2003, Divall et al. 2010, Miller and Gore. 2001, Daftary and Gore. 2004). IGF-1 of both peripheral and central origin can interact with hypothalamic IGF-1Rs and levels of IGF-1 in the brain have been shown to be important for puberty, estrous cyclicity, the preovulatory gonadotropin surge and the decline of reproductive neuronendocrine function during reproductive senescence. Hypothalamic IGF-1 expression has been shown to increase at puberty in mice (D’Ercole and Underwood. 1980), rats (Hiney et al. 1996, Frystyk et al. 1998), monkey (Suter et al. 2000) and human (Luna et al. 1983). Hiney et al. demonstrated that ICV infusion of IGF-1 stimulated secretion of GnRH in prepubertal female rats and could advance the onset of puberty (Hiney et al. 1991, Hiney et al. 1996) although GnRH expression was not altered in IGF-1 treated rats as assessed by RNase protection assay analysis of RNA from hypothalamic tissue (Miller and Gore. 2001). A similar precocious puberty was observed in the female mouse following peripheral treatment with IGF-1 (Divall et al. 2010, Danilovich et al. 1999), although this effect exhibited a sexual dimorphism with males not demonstrating an advance of puberty with IGF-1 treatment (Divall et al. 2010). Conversely, central infusion of male prepubertal rats with IGF-1 antiserum resulted in a delay of pubertal development (Pazos et al. 1999) further implicating IGF-1 as contributing to the initiation of puberty.
Our laboratory has demonstrated a conclusive role for IGF-1 signaling in the GnRH neurons in the elaboration of puberty (Divall et al. 2010). Mice lacking the IGF-1R in GnRH neurons (GnRH-IGF-1RKO) exhibited a delay in the onset of puberty (males, ~3 days, females, ~4 days), but did not exhibit impaired reproductive function as adults. These results are very similar to those observed in the kisspeptin-IRKO mouse (Qiu et al. 2013) underscoring the diversity of signals that likely contribute to the pubertal process. One striking finding from the analysis of the GnRH-IGF-1RKO mice was that there appeared to be a delay in the development of GnRH neurons to an adult morphology. Studies from the Hoffman laboratory indicated that during postnatal development, rodent GnRH neurons undergo changes in the percentage of cells with spiny processes; the percentage of total neurons with spines is lowest at birth and increases gradually postnatally until puberty (Wray and Hoffman. 1986) Additionally, the percentage of GnRH neurons that exhibit a complex dendritic structure decreases during postnatal development, reaching the low adult levels by puberty (Cottrell et al. 2006). Neurons with complex dendritic structures, and smooth dendritic processes were more abundant at day of life 10 in GnRH-IGF-1RKO mice than in WT mice (Divall et al. 2010) but these parameters are equivalent between GnRH-IGF-1RKO and WT mice by puberty. IGF-1R signaling in neurons is important for neuronal progenitor proliferation, neurite outgrowth, and synaptogenesis that is necessary for interneuronal communication (Joseph D’Ercole and Ye. 2008). These data strongly suggest that IGF-1 contributes to the timing of puberty by receptor mediated regulation of morphological development of GnRH neurons that is required for the complex, intercellular communication required for pubertal onset.
While the effects of IGF-1 signaling at the level of the GnRH neuron do not appear to be required for normal reproductive function in adults, a vast literature exists confirming an important role for hypothalamic IGF-1 signaling in maintaining healthy female reproductive cyclicity and specifically the elaboration of the preovulatory gonadotropin surge. Reduced levels of hypothalamic IGF-1 have been proposed to underlie the decline in neuroendocrine function associated with reproductive aging. Work from the Gore laboratory demonstrated that while there was an increase in hypothalamic IGF-1 levels during the pubertal progression, there was a significant reduction in levels of hypothalamic IGF-1 in older rats undergoing reproductive senescence (Miller and Gore. 2001). Work from the Etgen and Neal-Perry labs has extended these findings and demonstrated a requirement for hypothalamic IGF-1 signaling for proper timing and magnitude of the estradiol induction of the GnRH surge (Todd et al. 2010, Sun et al. 2011) and further supported a model in which reproductive senescence is due, in part, to reduced hypothalamic IGF-1 levels in middle aged rats. Interestingly, in light of the developmental role proposed for IGF-1 in puberty (Divall et al. 2010), IGF-1 may contribute to the regulation of synaptic remodeling of noradrenergic receptors in the hypothalamus in response to estradiol during the estrous cycle (Quesada and Etgen. 2002). Recent work from the Goya laboratory cements a role for IGF-1 signaling in the maintenance of estrous cyclicity in rats, and demonstrates that declines in IGF-1 contribute to impaired estrous cyclicity in older rats (Rodriguez et al. 2013). The authors used an adenoviral vector to deliver an IGF-I-expressing transgene to the mediobasal hypothalamus of middle-aged rats that normally exhibit impaired patterns of estrous cycling and showed that estrous cyclicity was maintained at ages where estrous cyclicity usually declines.
While direct effects of IGF-1 at the level of the GnRH neuron has been demonstrated for the regulation of puberty, some of the effects of IGF-1 on GnRH neuronal function could be indirectly mediated at the level of the kisspeptin neuron. Studies from Hiney et al. have demonstrated that IGF-1 stimulated Kiss1 expression in the hypothalamus. Both centrally perfused IGF-1 and peripherally injected IGF-1 stimulated the kisspeptin neurons of the AVPV (Hiney et al. 2009). These data provide another potential locus of action for IGF-1 regulation of reproductive function. They may also provide an anatomical explanation for the sexual dimorphism in the response of mice to IGF-1since males have only a poorly defined AVPV population of Kiss1 neurons.
7.2. IGF-1 regulation of pituitary gonadotroph
There is evidence for a functional role for IGF-1R in the pituitary gonadotroph. Both the pituitary gonadotroph and gonadotroph cell models such as the LβT2 and αT3-1 cell lines express IGF-1R (Long and Buggs. 2008, Bach and Bondy. 1992, Rose et al. 2004, Mutiara et al. 2008, Unger and Lange. 1997). Studies in αT3-1 cells, which express both Esr1 and Igf1r, describe an IGF-1 stimulated increase in mitogenic activity (Eertmans et al. 2007) and proposes a cross talk between IGF-1 and ESR1 signaling pathways with IGF-1 enhancing both basal and estradiol stimulated gene expression.
Several studies have reported that IGF-1 stimulates LH synthesis and /or secretion (Soldani et al. 1994, Kanematsu et al. 1991, Whitley et al. 1995, Soldani et al. 1995, Hashizume et al. 2002, Xia et al. 2001) although one study found that while IGF-1 activated PI3K signaling in LβT2 cells (Mutiara et al. 2008), no role was observed for IGF-1R activation in regulating gene expression of Lhb, Fshb or the Cga genes and this signaling pathway may regulate LH secretion (Weiss et al. 2003). These effects may not mediate acute changes in IGF-1 levels since one study found no effect of short term IGF-1 treatment on LH synthesis and release (Weiss et al. 2006) and noted that effects of IGF-I on LH-secretion in female rat pituitary cells require long-term treatment (Xia et al. 2001). As noted by this author and others, the effect of IGF-1 on pituitary cell cultures depends upon the nature of the pituitary cell culture and the culture conditions. The lack of consistent findings suggests that the IGF receptor or its intracellular signaling pathway may be altered by isolation and culture of anterior pituitary cells.
Conclusions
Our understanding of the role of insulin and IGF-1 signaling in the hypothalamic-pituitary regulation of reproduction have been aided by the development of GnRH neuron and pituitary gonadotroph cell lines which allow for the direct examination of signaling pathways mediating the effects of insulin and IGF-1. Additionally, the use of the CRE/LoxP system to target disruption of signaling to specific components of the axiscan reveal the role these receptors play in development, physiology and in pathophysiology. The studies reviewed here have revealed fundamental clues to the role these growth factors play, but much remains to be discovered.
Insulin appears to exert effects both in the brain and at the pituitary. While insulin signaling at the level of the GnRH neuron does not appear to impact puberty and fertility under normal physiological and nutritional conditions, insulin signaling in the Kiss1 neurons may be one of a number of signals contributing to the timing of puberty. In states of chronic high circulating insulin levels, reproductive dysfunction appears to be mediated by insulin signaling at the level of the pituitary gonadotroph and at the level of the ovarian theca cell (In press (Wu et al. 2013) suggesting this may represent a common mechanism in the reproductive hormone axis for the pathophysiological effects of obesity. Future studies could include an exploration of the effects of high insulin levels directly at the level of the GnRH or Kiss1 neurons.
The consensus seems to be clear that IGF-1 contributes to the timing of puberty in males and females via effects at the level of the GnRH neuron and possibly the Kiss1 neuron. However, many of the studies highlighted here make clear that the nutritional regulation of puberty is a complex, multifactorial process and will require additional animal models to tease apart the regulatory inputs to the GnRH neurons. Directing the development of these models may be genetic discovery and system biology studies revealing new signaling pathways, neuronal connectivity, glial input and transcription factors. Additionally, a significant deficit exists in our understanding of IGF-1 signaling in the pituitary gonadotroph. The field awaits the production of a conditional KO model.
It is not surprising that the nutritional regulation of puberty and reproductive function involves multiple contributing factors and all components of the reproductive neuronal hierarchy. The ability to support a fetus through gestation is a nutritionally demanding endeavor, thus an axis that is exquisitely sensitive to environmental nutritional cues is necessary for species survival.
Kisspeptin neuron, GnRH neuron and pituitary gonadotrophs are targets of insulin and IGF-1 action
Insulin action in the pituitary may mediate pathophysiologic effects of obesity
IGF-1 in the brain may act at both the kisspeptin and GnRH neurons to regulate puberty
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Abraham IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Martinez M, Luo J, Elias C, Wolfe A, Levine JE. Male-biased effects of gonadotropin-releasing hormone neuron-specific deletion of the phosphoinositide 3-kinase regulatory subunit p85alpha on the reproductive axis. Endocrinology. 2009;150:4203–4212. doi: 10.1210/en.2008-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman JP, Mason AJ, Hayflick JS, Seeburg PH. Isolation of the gene and hypothalamic cDNA for the common precursor of gonadotropin-releasing hormone and prolactin release-inhibiting factor in human and rat. Proc Natl Acad Sci U S A. 1986;83:179–183. doi: 10.1073/pnas.83.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima RS, Dushay J, Flier SN, Prabakaran D, Flier JS. Leptin accelerates the onset of puberty in normal female mice. J Clin Invest. 1997;99:391–395. doi: 10.1172/JCI119172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- Alarid ET, Windle JJ, Whyte DB, Mellon PL. Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development. 1996;122:3319–3329. doi: 10.1242/dev.122.10.3319. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Zwain IH, Arroyo A, Mellon PL, Yen SS. The insulin-like growth factor system in the GT1-7 GnRH neuronal cell line. Neuroendocrinology. 1999;70:353–359. doi: 10.1159/000054496. [DOI] [PubMed] [Google Scholar]
- Andrews WW, Advis JP, Ojeda SR. The maturation of estradiol-negative feedback in female rats: evidence that the resetting of the hypothalamic “gonadostat” does not precede the first preovulatory surge of gonadotropins. Endocrinology. 1981;109:2022–2031. doi: 10.1210/endo-109-6-2022. [DOI] [PubMed] [Google Scholar]
- Apter D, Butzow T, Laughlin GA, Yen SS. Accelerated 24-hour luteinizing hormone pulsatile activity in adolescent girls with ovarian hyperandrogenism: relevance to the developmental phase of polycystic ovarian syndrome. J Clin Endocrinol Metab. 1994;79:119–125. doi: 10.1210/jcem.79.1.8027216. [DOI] [PubMed] [Google Scholar]
- Arriola DJ, Mayo SL, Skarra DV, Benson CA, Thackray VG. FOXO1 transcription factor inhibits luteinizing hormone beta gene expression in pituitary gonadotrope cells. J Biol Chem. 2012;287:33424–33435. doi: 10.1074/jbc.M112.362103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach MA, Bondy CA. Anatomy of the pituitary insulin-like growth factor system. Endocrinology. 1992;131:2588–2594. doi: 10.1210/endo.131.6.1280202. [DOI] [PubMed] [Google Scholar]
- Baker J, Hardy MP, Zhou J, Bondy C, Lupu F, Bellve AR, Efstratiadis A. Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol. 1996;10:903–918. doi: 10.1210/mend.10.7.8813730. [DOI] [PubMed] [Google Scholar]
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- Bakker J, Kelliher KR, Baum MJ. Mating induces gonadotropin-releasing hormone neuronal activation in anosmic female ferrets. Biol Reprod. 2001;64:1100–1105. doi: 10.1095/biolreprod64.4.1100. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay G, Standaert ML, Zhao L, Yu B, Avignon A, Galloway L, Karnam P, Moscat J, Farese RV. Activation of protein kinase C (alpha, beta, and zeta) by insulin in 3T3/L1 cells. Transfection studies suggest a role for PKC-zeta in glucose transport. J Biol Chem. 1997;272:2551–2558. doi: 10.1074/jbc.272.4.2551. [DOI] [PubMed] [Google Scholar]
- Baskin DG, Woods SC, West DB, van Houten M, Posner BI, Dorsa DM, Porte D., Jr Immunocytochemical detection of insulin in rat hypothalamus and its possible uptake from cerebrospinal fluid. Endocrinology. 1983;113:1818–1825. doi: 10.1210/endo-113-5-1818. [DOI] [PubMed] [Google Scholar]
- Baura GD, Foster DM, Porte D, Jr, Kahn SE, Bergman RN, Cobelli C, Schwartz MW. Saturable transport of insulin from plasma into the central nervous system of dogs in vivo. A mechanism for regulated insulin delivery to the brain. J Clin Invest. 1993;92:1824–1830. doi: 10.1172/JCI116773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter RC, Martin JL, Beniac VA. High molecular weight insulin-like growth factor binding protein complex. Purification and properties of the acid-labile subunit from human serum. J Biol Chem. 1989;264:11843–11848. [PubMed] [Google Scholar]
- Benyoucef S, Surinya KH, Hadaschik D, Siddle K. Characterization of insulin/IGF hybrid receptors: contributions of the insulin receptor L2 and Fn1 domains and the alternatively spliced exon 11 sequence to ligand binding and receptor activation. Biochem J. 2007;403:603–613. doi: 10.1042/BJ20061709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berga SL, Daniels TL, Giles DE. Women with functional hypothalamic amenorrhea but not other forms of anovulation display amplified cortisol concentrations. Fertil Steril. 1997;67:1024–1030. doi: 10.1016/s0015-0282(97)81434-3. [DOI] [PubMed] [Google Scholar]
- Berga SL, Mortola JF, Girton L, Suh B, Laughlin G, Pham P, Yen SS. Neuroendocrine aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 1989;68:301–308. doi: 10.1210/jcem-68-2-301. [DOI] [PubMed] [Google Scholar]
- Besecke LM, Wolfe AM, Pierce ME, Takahashi JS, Levine JE. Neuropeptide Y stimulates luteinizing hormone-releasing hormone release from superfused hypothalamic GT1-7 cells. Endocrinology. 1994;135(4):1621–1627. doi: 10.1210/endo.135.4.7925125. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Lima FB, Centeno ML, Weissheimer KV, Senashova O, Reddy AP, Cameron JL. Effects of citalopram on serotonin and CRF systems in the midbrain of primates with differences in stress sensitivity. J Chem Neuroanat. 2011;41:200–218. doi: 10.1016/j.jchemneu.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienvenu G, Seurin D, Grellier P, Froment P, Baudrimont M, Monget P, Le Bouc Y, Babajko S. Insulin-like growth factor binding protein-6 transgenic mice: postnatal growth, brain development, and reproduction abnormalities. Endocrinology. 2004;145:2412–2420. doi: 10.1210/en.2003-1196. [DOI] [PubMed] [Google Scholar]
- Bliss SP, Miller A, Navratil AM, Xie J, McDonough SP, Fisher PJ, Landreth GE, Roberson MS. ERK signaling in the pituitary is required for female but not male fertility. Mol Endocrinol. 2009;23:1092–1101. doi: 10.1210/me.2009-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogovich K, Richards JS. Androgen biosynthesis in developing ovarian follicles: evidence that luteinizing hormone regulates thecal 17 alpha-hydroxylase and C17-20-lyase activities. Endocrinology. 1982;111:1201–1208. doi: 10.1210/endo-111-4-1201. [DOI] [PubMed] [Google Scholar]
- Bond CT, Hayflick JS, Seeburg PH, Adelman JP. The rat gonadotropin-releasing hormone: SH locus: structure and hypothalamic expression. Mol Endo. 1989;3(8):1257–1262. doi: 10.1210/mend-3-8-1257. [DOI] [PubMed] [Google Scholar]
- Bosma MM. Ion channel properties and episodic activity in isolated immortalized gonadotropin-releasing hormone (GnRH) neurons. J Membr Biol. 1993;136:85–96. doi: 10.1007/BF00241492. [DOI] [PubMed] [Google Scholar]
- Boukhliq R, Martin GB. Administration of fatty acids and gonadotrophin secretion in the mature ram. Anim Reprod Sci. 1997;49:143–159. doi: 10.1016/s0378-4320(97)00065-1. [DOI] [PubMed] [Google Scholar]
- Brothers KJ, Wu S, DiVall SA, Messmer MR, Kahn CR, Miller RS, Radovick S, Wondisford FE, Wolfe A. Rescue of obesity-induced infertility in female mice due to a pituitary-specific knockout of the insulin receptor. Cell Metab. 2010;12:295–305. doi: 10.1016/j.cmet.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Buggs C, Weinberg F, Kim E, Wolfe A, Radovick S, Wondisford F. Insulin augments GnRH-stimulated LHbeta gene expression by Egr-1. Mol Cell Endocrinol. 2006;249:99–106. doi: 10.1016/j.mce.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunting M, Bernstein KE, Greer JM, Capecchi MR, Thomas KR. Targeting genes for self-excision in the germ line. Genes Dev. 1999;13:1524–1528. doi: 10.1101/gad.13.12.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Bentsen AH, Mikkelsen JD, Tena-Sempere M. Kisspeptins: bridging energy homeostasis and reproduction. Brain Res. 2010a;1364:129–138. doi: 10.1016/j.brainres.2010.08.057. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Bentsen AH, Romero M, Pineda R, Ruiz-Pino F, Garcia-Galiano D, Sanchez-Garrido MA, Pinilla L, Mikkelsen JD, Tena-Sempere M. Acute inflammation reduces kisspeptin immunoreactivity at the arcuate nucleus and decreases responsiveness to kisspeptin independently of its anorectic effects. Am J Physiol Endocrinol Metab. 2010b;299:E54–61. doi: 10.1152/ajpendo.00081.2010. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Fernandez-Fernandez R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar E, et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–3925. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Fernandez-Fernandez R, Roa J, Vigo E, Pineda R, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Expression of hypothalamic KiSS-1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocin-induced diabetic male rats. Diabetes. 2006;55:2602–2610. doi: 10.2337/db05-1584. [DOI] [PubMed] [Google Scholar]
- Cates PS, O’Byrne KT. The area postrema mediates insulin hypoglycaemia-induced suppression of pulsatile LH secretion in the female rat. Brain Res. 2000;853:151–155. doi: 10.1016/s0006-8993(99)02301-x. [DOI] [PubMed] [Google Scholar]
- Centeno ML, Sanchez RL, Cameron JL, Bethea CL. Hypothalamic expression of serotonin 1A, 2A and 2C receptor and GAD67 mRNA in female cynomolgus monkeys with different sensitivity to stress. Brain Res. 2007a;1142:1–12. doi: 10.1016/j.brainres.2007.01.056. [DOI] [PubMed] [Google Scholar]
- Centeno ML, Sanchez RL, Cameron JL, Bethea CL. Hypothalamic gonadotrophin-releasing hormone expression in female monkeys with different sensitivity to stress. J Neuroendocrinol. 2007b;19:594–604. doi: 10.1111/j.1365-2826.2007.01566.x. [DOI] [PubMed] [Google Scholar]
- Charles MA, Mortensen AH, Potok MA, Camper SA. Pitx2 deletion in pituitary gonadotropes is compatible with gonadal development, puberty, and fertility. Genesis. 2008;46:507–514. doi: 10.1002/dvg.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehab FF, Mounzih K, Lu R, Lim ME. Early onset of reproductive function in normal female mice treated with leptin. Science. 1997;275:88–90. doi: 10.1126/science.275.5296.88. [DOI] [PubMed] [Google Scholar]
- Cheung CC, Thornton JE, Kuijper JL, Weigle DS, Clifton DK, Steiner RA. Leptin is a metabolic gate for the onset of puberty in the female rat. Endocrinology. 1997;138:855–858. doi: 10.1210/endo.138.2.5054. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons DR, Klibanski A, Underwood LE, McArthur JW, Ridgway EC, Beitins IZ, Van Wyk JJ. Reduction of plasma immunoreactive somatomedin C during fasting in humans. J Clin Endocrinol Metab. 1981;53:1247–1250. doi: 10.1210/jcem-53-6-1247. [DOI] [PubMed] [Google Scholar]
- Colledge WH. GPR54 and kisspeptins. Results Probl Cell Differ. 2008;46:117–143. doi: 10.1007/400_2007_050. [DOI] [PubMed] [Google Scholar]
- Cooke RM, Harvey TS, Campbell ID. Solution structure of human insulin-like growth factor 1: a nuclear magnetic resonance and restrained molecular dynamics study. Biochemistry. 1991;30:5484–5491. doi: 10.1021/bi00236a022. [DOI] [PubMed] [Google Scholar]
- Coolican SA, Samuel DS, Ewton DZ, McWade FJ, Florini JR. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem. 1997;272:6653–6662. doi: 10.1074/jbc.272.10.6653. [DOI] [PubMed] [Google Scholar]
- Copeland KC, Kuehl TJ, Castracane VD. Pubertal endocrinology of the baboon: elevated somatomedin-C/insulin-like growth factor I at puberty. J Clin Endocrinol Metab. 1982;55:1198–1201. doi: 10.1210/jcem-55-6-1198. [DOI] [PubMed] [Google Scholar]
- Corp ES, Woods SC, Porte D, Jr, Dorsa DM, Figlewicz DP, Baskin DG. Localization of 125I-insulin binding sites in the rat hypothalamus by quantitative autoradiography. Neurosci Lett. 1986;70:17–22. doi: 10.1016/0304-3940(86)90430-1. [DOI] [PubMed] [Google Scholar]
- Cottrell EC, Campbell RE, Han SK, Herbison AE. Postnatal remodeling of dendritic structure and spine density in gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:3652–3661. doi: 10.1210/en.2006-0296. [DOI] [PubMed] [Google Scholar]
- Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J, Jr, Atkin S, Bookout AL, Rovinsky S, Frazao R, Lee CE, Gautron L, et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37–56. doi: 10.1016/j.neuroscience.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley WF. The developmental biology of the GnRH neurons. Mol Cell Endocrinol. 2011;346:1–3. doi: 10.1016/j.mce.2011.06.023. [DOI] [PubMed] [Google Scholar]
- Cushman LJ, Burrows HL, Seasholtz AF, Lewandoski M, Muzyczka N, Camper SA. Cre-mediated recombination in the pituitary gland. Genesis. 2000;28:167–174. doi: 10.1002/1526-968x(200011/12)28:3/4<167::aid-gene120>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Czech MP, Corvera S. Signaling mechanisms that regulate glucose transport. J Biol Chem. 1999;274:1865–1868. doi: 10.1074/jbc.274.4.1865. [DOI] [PubMed] [Google Scholar]
- Daftary SS, Gore AC. The hypothalamic insulin-like growth factor-1 receptor and its relationship to gonadotropin-releasing hormones neurones during postnatal development. J Neuroendocrinol. 2004;16:160–169. doi: 10.1111/j.0953-8194.2004.01149.x. [DOI] [PubMed] [Google Scholar]
- Daftary SS, Gore AC. Developmental changes in hypothalamic insulin-like growth factor-1: relationship to gonadotropin-releasing hormone neurons. Endocrinology. 2003;144:2034–2045. doi: 10.1210/en.2002-221025. [DOI] [PubMed] [Google Scholar]
- Danilovich N, Wernsing D, Coschigano KT, Kopchick JJ, Bartke A. Deficits in female reproductive function in GH-R-KO mice; role of IGF-I. Endocrinology. 1999;140:2637–2640. doi: 10.1210/endo.140.6.6992. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci U S A. 2004;101:1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ercole AJ, Underwood LE. Ontogeny of somatomedin during development in the mouse. Serum concentrations, molecular forms, binding proteins, and tissue receptors. Dev Biol. 1980;79:33–45. doi: 10.1016/0012-1606(80)90071-8. [DOI] [PubMed] [Google Scholar]
- D’Ercole AJ, Ye P, Calikoglu AS, Gutierrez-Ospina G. The role of the insulin-like growth factors in the central nervous system. Mol Neurobiol. 1996;13:227–255. doi: 10.1007/BF02740625. [DOI] [PubMed] [Google Scholar]
- Diaczok D, Divall S, Matsuo I, Wondisford FE, Wolfe AM, Radovick S. Deletion of otx2 in GnRH neurons results in a mouse model of hypogonadotropic hypogonadism. Mol Endocrinol. 2011;25:833–846. doi: 10.1210/me.2010-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiVall SA, Radovick S, Wolfe A. Egr-1 binds the GnRH promoter to mediate the increase in gene expression by insulin. Mol Cell Endocrinol. 2007;270:64–72. doi: 10.1016/j.mce.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divall SA, Williams TR, Carver SE, Koch L, Bruning JC, Kahn CR, Wondisford F, Radovick S, Wolfe A. Divergent roles of growth factors in the GnRH regulation of puberty in mice. J Clin Invest. 2010;120:2900–2909. doi: 10.1172/JCI41069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato J, Jr, Cravo RM, Frazao R, Gautron L, Scott MM, Lachey J, Castro IA, Margatho LO, Lee S, Lee C, et al. Leptin’s effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest. 2011;121:355–368. doi: 10.1172/JCI45106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong KW, Duval P, Zeng Z, Gordon K, Williams RF, Hodgen GD, Jones G, Kerdelhue B, Roberts JL. Multiple transcription start sites for the GnRH gene in rhesus and cynomolgus monkeys: a non-human primate model for studying GnRH gene regulation. Mol Cell Endocrinol. 1996;117:121–130. doi: 10.1016/0303-7207(95)03737-3. [DOI] [PubMed] [Google Scholar]
- Dong Q, Lazarus RM, Wong LS, Vellios M, Handelsman DJ. Pulsatile LH secretion in streptozotocin-induced diabetes in the rat. J Endocrinol. 1991;131:49–55. doi: 10.1677/joe.0.1310049. [DOI] [PubMed] [Google Scholar]
- Dorn C, Mouillet JF, Yan X, Ou Q, Sadovsky Y. Insulin enhances the transcription of luteinizing hormone-beta gene. Am J Obstet Gynecol. 2004;191:132–137. doi: 10.1016/j.ajog.2004.01.054. [DOI] [PubMed] [Google Scholar]
- Eertmans F, Dhooge W, De Wever O, Bracke M, Comhaire F, Kaufman JM. Estrogen receptor alpha (ERalpha) and insulin-like growth factor I receptor (IGF-IR) cross-talk in the gonadotropic alphaT3-1 cell line. J Cell Physiol. 2007;212:583–590. doi: 10.1002/jcp.21053. [DOI] [PubMed] [Google Scholar]
- Egawa K, Maegawa H, Shi K, Nakamura T, Obata T, Yoshizaki T, Morino K, Shimizu S, Nishio Y, Suzuki E, et al. Membrane localization of 3-phosphoinositide-dependent protein kinase-1 stimulates activities of Akt and Atypical PKC, but does not stimulate glucose transport and glycogen synthesis in 3T3-L1 adipocytes. J Biol Chem. 2002 doi: 10.1074/jbc.M203132200. [DOI] [PubMed] [Google Scholar]
- Elamin A, Hussein O, Tuvemo T. Growth, puberty, and final height in children with Type 1 diabetes. J Diabetes Complications. 2006;20:252–256. doi: 10.1016/j.jdiacomp.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fernandez R, Tena-Sempere M, Navarro VM, Barreiro ML, Castellano JM, Aguilar E, Pinilla L. Effects of ghrelin upon gonadotropin-releasing hormone and gonadotropin secretion in adult female rats: in vivo and in vitro studies. Neuroendocrinology. 2005;82:245–255. doi: 10.1159/000092753. [DOI] [PubMed] [Google Scholar]
- Ferris HA, Shupnik MA. Mechanisms for pulsatile regulation of the gonadotropin subunit genes by GNRH1. Biol Reprod. 2006;74:993–998. doi: 10.1095/biolreprod.105.049049. [DOI] [PubMed] [Google Scholar]
- Forbes GB, Brown MR, Welle SL, Underwood LE. Hormonal response to overfeeding. Am J Clin Nutr. 1989;49:608–611. doi: 10.1093/ajcn/49.4.608. [DOI] [PubMed] [Google Scholar]
- Frazao R, Cravo RM, Donato J, Jr, Ratra DV, Clegg DJ, Elmquist JK, Zigman JM, Williams KW, Elias CF. Shift in Kiss1 cell activity requires estrogen receptor alpha. J Neurosci. 2013;33:2807–2820. doi: 10.1523/JNEUROSCI.1610-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froment P, Seurin D, Hembert S, Levine JE, Pisselet C, Monniaux D, Binoux M, Monget P. Reproductive abnormalities in human IGF binding protein-1 transgenic female mice. Endocrinology. 2002;143:1801–1808. doi: 10.1210/endo.143.5.8815. [DOI] [PubMed] [Google Scholar]
- Froment P, Staub C, Hembert S, Pisselet C, Magistrini M, Delaleu B, Seurin D, Levine JE, Johnson L, Binoux M, et al. Reproductive abnormalities in human insulin-like growth factor-binding protein-1 transgenic male mice. Endocrinology. 2004;145:2080–2091. doi: 10.1210/en.2003-0956. [DOI] [PubMed] [Google Scholar]
- Frystyk J, Delhanty PJ, Skjaerbaek C, Baxter RC. Changes in the circulating IGF system during short-term fasting and refeeding in rats. Am J Physiol. 1999;277:E245–52. doi: 10.1152/ajpendo.1999.277.2.E245. [DOI] [PubMed] [Google Scholar]
- Frystyk J, Gronbaek H, Skjaerbaek C, Flyvbjerg A, Orskov H, Baxter RC. Developmental changes in serum levels of free and total insulin-like growth factor I (IGF-I), IGF-binding protein-1 and -3, and the acid-labile subunit in rats. Endocrinology. 1998;139:4286–4292. doi: 10.1210/endo.139.10.6273. [DOI] [PubMed] [Google Scholar]
- Frystyk J, Skjaerbaek C, Vestbo E, Fisker S, Orskov H. Circulating levels of free insulin-like growth factors in obese subjects: the impact of type 2 diabetes. Diabetes Metab Res Rev. 1999;15:314–322. doi: 10.1002/(sici)1520-7560(199909/10)15:5<314::aid-dmrr56>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Gamba M, Pralong FP. Control of GnRH neuronal activity by metabolic factors: the role of leptin and insulin. Mol Cell Endocrinol. 2006;254–255:133–139. doi: 10.1016/j.mce.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Gauguin L, Klaproth B, Sajid W, Andersen AS, McNeil KA, Forbes BE, De Meyts P. Structural basis for the lower affinity of the insulin-like growth factors for the insulin receptor. J Biol Chem. 2008;283:2604–2613. doi: 10.1074/jbc.M709220200. [DOI] [PubMed] [Google Scholar]
- Giacobini P, Wray S. Cholecystokinin directly inhibits neuronal activity of primary gonadotropin-releasing hormone cells through cholecystokinin-1 receptor. Endocrinology. 2007;148:63–71. doi: 10.1210/en.2006-0758. [DOI] [PubMed] [Google Scholar]
- Gieske MC, Kim HJ, Legan SJ, Koo Y, Krust A, Chambon P, Ko C. Pituitary gonadotroph estrogen receptor-alpha is necessary for fertility in females. Endocrinology. 2008;149:20–27. doi: 10.1210/en.2007-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles DE, Berga SL. Cognitive and psychiatric correlates of functional hypothalamic amenorrhea: a controlled comparison. Fertil Steril. 1993;60:486–492. [PubMed] [Google Scholar]
- Giovannone B, Scaldaferri ML, Federici M, Porzio O, Lauro D, Fusco A, Sbraccia P, Borboni P, Lauro R, Sesti G. Insulin receptor substrate (IRS) transduction system: distinct and overlapping signaling potential. Diabetes Metab Res Rev. 2000;16:434–441. doi: 10.1002/1520-7560(2000)9999:9999<::aid-dmrr159>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Hileman SM, Nestor CC, Porter KL, Connors JM, Hardy SL, Millar RP, Cernea M, Coolen LM, Lehman MN. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology. 2013;154:4259–4269. doi: 10.1210/en.2013-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsch ML, Clifton DK, Steiner RA. Kisspepeptin-GPR54 signaling in the neuroendocrine reproductive axis. Mol Cell Endocrinol. 2006;254–255:91–96. doi: 10.1016/j.mce.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Popa SM, Lawhorn JK, Qiu J, Tonsfeldt KJ, Bosch MA, Kelly MJ, Ronnekleiv OK, Sanz E, McKnight GS, et al. Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology. 2011;152:4298–4309. doi: 10.1210/en.2011-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham KE, Nusser KD, Low MJ. LbetaT2 gonadotroph cells secrete follicle stimulating hormone (FSH) in response to active A. J Endocrinol. 1999;162:R1–5. doi: 10.1677/joe.0.162r001. [DOI] [PubMed] [Google Scholar]
- Greives TJ, Mason AO, Scotti MA, Levine J, Ketterson ED, Kriegsfeld LJ, Demas GE. Environmental control of kisspeptin: implications for seasonal reproduction. Endocrinology. 2007;148:1158–1166. doi: 10.1210/en.2006-1249. [DOI] [PubMed] [Google Scholar]
- Gu GB, Simerly RB. Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. J Comp Neurol. 1997;384:142–164. [PubMed] [Google Scholar]
- Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez S, Mukdsi JH, Aoki A, Torres AI, Soler AP, Orgnero EM. Ultrastructural immunolocalization of IGF-1 and insulin receptors in rat pituitary culture: evidence of a functional interaction between gonadotroph and lactotroph cells. Cell Tissue Res. 2007;327:121–132. doi: 10.1007/s00441-006-0283-4. [DOI] [PubMed] [Google Scholar]
- Haisenleder DJ, Dalkin AC, Ortolano GA, Marshall JC, Shupnik MA. A pulsatile gonadotropin-releasing hormone stimulus is required to increase transcription of the gonadotropin subunit genes: evidence for differential regulation of transcription by pulse frequency in vivo. Endocrinology. 1991;128:509–517. doi: 10.1210/endo-128-1-509. [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume T, Kumahara A, Fujino M, Okada K. Insulin-like growth factor I enhances gonadotropin-releasing hormone-stimulated luteinizing hormone release from bovine anterior pituitary cells. Anim Reprod Sci. 2002;70:13–21. doi: 10.1016/s0378-4320(01)00190-7. [DOI] [PubMed] [Google Scholar]
- Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978;272:827–829. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- Hawes BE, Barnes S, Conn PM. Cholera toxin and pertussis toxin provoke differential effects on luteinizing hormone release, inositol phosphate production, and gonadotropin-releasing hormone (GnRH) receptor binding in the gonadotrope: evidence for multiple guanyl nucleotide binding proteins in GnRH action. Endocrinology. 1993;132:2124–2130. doi: 10.1210/endo.132.5.8386608. [DOI] [PubMed] [Google Scholar]
- Hayes FJ, Taylor AE, Martin KA, Hall JE. Use of a gonadotropin-releasing hormone antagonist as a physiologic probe in polycystic ovary syndrome: assessment of neuroendocrine and androgen dynamics. J Clin Endocrinol Metab. 1998;83:2343–2349. doi: 10.1210/jcem.83.7.4925. [DOI] [PubMed] [Google Scholar]
- Hayflick JS, Adelman JP, Seeburg PH. The complete nucleotide sequence of the human gonadotropin- releasing hormone gene. Nucleic Acid Res. 1989;17(15):6403–6404. doi: 10.1093/nar/17.15.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D, Funabashi T, Sano A, Uemura T, Minaguchi H, Kimura F. Effects of glucose and related substrates on the recovery of the electrical activity of gonadotropin-releasing hormone pulse generator which is decreased by insulin-induced hypoglycemia in the estrogen- primed ovariectomized rat. Brain Res. 1999;820:71–76. doi: 10.1016/s0006-8993(98)01358-4. [DOI] [PubMed] [Google Scholar]
- Hedin L, Rodgers RJ, Simpson ER, Richards JS. Changes in content of cytochrome P450(17)alpha, cytochrome P450scc, and 3-hydroxy-3-methylglutaryl CoA reductase in developing rat ovarian follicles and corpora lutea: correlation with theca cell steroidogenesis. Biol Reprod. 1987;37:211–223. doi: 10.1095/biolreprod37.1.211. [DOI] [PubMed] [Google Scholar]
- Helmreich DL, Cameron JL. Suppression of luteinizing hormone secretion during food restriction in male rhesus monkeys (Macaca mulatta): failure of naloxone to restore normal pulsatility. Neuroendocrinology. 1992;56:464–473. doi: 10.1159/000126263. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Moenter SM. Depolarising and hyperpolarising actions of GABA(A) receptor activation on gonadotrophin-releasing hormone neurones: towards an emerging consensus. J Neuroendocrinol. 2011;23:557–569. doi: 10.1111/j.1365-2826.2011.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herde MK, Geist K, Campbell RE, Herbison AE. Gonadotropin-releasing hormone neurons extend complex highly branched dendritic trees outside the blood-brain barrier. Endocrinology. 2011;152:3832–3841. doi: 10.1210/en.2011-1228. [DOI] [PubMed] [Google Scholar]
- Herod SM, Dettmer AM, Novak MA, Meyer JS, Cameron JL. Sensitivity to stress-induced reproductive dysfunction is associated with a selective but not a generalized increase in activity of the adrenal axis. Am J Physiol Endocrinol Metab. 2011a;300:E28–36. doi: 10.1152/ajpendo.00223.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herod SM, Pohl CR, Cameron JL. Treatment with a CRH-R1 antagonist prevents stress-induced suppression of the central neural drive to the reproductive axis in female macaques. Am J Physiol Endocrinol Metab. 2011b;300:E19–27. doi: 10.1152/ajpendo.00224.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier SG, Reichert LE, Jr, Van Hall EV. Control of preovulatory follicular estrogen biosynthesis in the human ovary. J Clin Endocrinol Metab. 1981;52:847–856. doi: 10.1210/jcem-52-5-847. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Ojeda SR, Dees WL. Insulin-like growth factor I: a possible metabolic signal involved in the regulation of female puberty. Neuroendocrinology. 1991;54:420–423. doi: 10.1159/000125924. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Srivastava V, Nyberg CL, Ojeda SR, Dees WL. Insulin-like growth factor I of peripheral origin acts centrally to accelerate the initiation of female puberty. Endocrinology. 1996;137:3717–3728. doi: 10.1210/endo.137.9.8756538. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Srivastava VK, Pine MD, Les Dees W. Insulin-like growth factor-I activates KiSS-1 gene expression in the brain of the prepubertal female rat. Endocrinology. 2009;150:376–384. doi: 10.1210/en.2008-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- Huang W, Acosta-Martinez M, Horton TH, Levine JE. Fasting-induced suppression of LH secretion does not require activation of ATP-sensitive potassium channels. Am J Physiol Endocrinol Metab. 2008;295:E1439–46. doi: 10.1152/ajpendo.90615.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez L, Potau N, Zampolli M, Rique S, Saenger P, Carrascosa A. Hyperinsulinemia and decreased insulin-like growth factor-binding protein-1 are common features in prepubertal and pubertal girls with a history of premature pubarche. J Clin Endocrinol Metab. 1997;82:2283–2288. doi: 10.1210/jcem.82.7.4084. [DOI] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- Jennes L, Stumpf WE. Gonadotropin-releasing hormone immunoreactive neurons with access to fenestrated capillaries in mouse brain. Neuroscience. 1986;18:403–416. doi: 10.1016/0306-4522(86)90162-4. [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Joseph D’Ercole A, Ye P. Expanding the mind: insulin-like growth factor I and brain development. Endocrinology. 2008;149:5958–5962. doi: 10.1210/en.2008-0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalamatianos T, Grimshaw SE, Poorun R, Hahn JD, Coen CW. Fasting reduces KiSS-1 expression in the anteroventral periventricular nucleus (AVPV): effects of fasting on the expression of KiSS-1 and neuropeptide Y in the AVPV or arcuate nucleus of female rats. J Neuroendocrinol. 2008;20:1089–1097. doi: 10.1111/j.1365-2826.2008.01757.x. [DOI] [PubMed] [Google Scholar]
- Kanematsu T, Irahara M, Miyake T, Shitsukawa K, Aono T. Effect of insulin-like growth factor I on gonadotropin release from the hypothalamus-pituitary axis in vitro. Acta Endocrinol (Copenh) 1991;125:227–233. doi: 10.1530/acta.0.1250227. [DOI] [PubMed] [Google Scholar]
- Kawaguchi M, Funabashi T, Kimura F. Insulin-induced hypoglycemia does not impair the surge of luteinizing hormone secretion in the proestrous rat. Neurosci Lett. 1998;256:131–134. doi: 10.1016/s0304-3940(98)00783-6. [DOI] [PubMed] [Google Scholar]
- Kazer RR, Kessel B, Yen SS. Circulating luteinizing hormone pulse frequency in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1987;65:233–236. doi: 10.1210/jcem-65-2-233. [DOI] [PubMed] [Google Scholar]
- Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149:4151–4157. doi: 10.1210/en.2008-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepa JK, Spaulding AJ, Jacobsen BM, Fang Z, Xiong X, Radovick S, Wierman ME. Structure of the distal human gonadotropin releasing hormone (hGnrh) gene promoter and functional analysis in Gt1-7 neuronal cells. Nucleic Acids Res. 1996;24:3614–3620. doi: 10.1093/nar/24.18.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepa JK, Wang C, Neeley CI, Raynolds MV, Gordon DF, Wood WM, Wierman ME. Structure of the rat gonadotropin-releasing hormone (rGnRH) gene promoter and functional analysis in hypothalamic cells. Nucleic Acid Res. 1992;20(6):1393–1399. doi: 10.1093/nar/20.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GL, Wang X, Chalmers JA, Thompson DR, Dhillon SS, Koletar MM, Belsham DD. Generation of immortal cell lines from the adult pituitary: role of cAMP on differentiation of SOX2-expressing progenitor cells to mature gonadotropes. PLoS One. 2011;6:e27799. doi: 10.1371/journal.pone.0027799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HH, Divall SA, Deneau RM, Wolfe A. Insulin regulation of GnRH gene expression through MAP kinase signaling pathways. Mol Cell Endocrinol. 2005;242:42–49. doi: 10.1016/j.mce.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kim HH, Wolfe A, Cohen RN, Eames SC, Johnson AL, Wieland CN, Radovick S. In vivo identification of a 107-base pair promoter element mediating neuron-specific expression of mouse gonadotropin-releasing hormone. Mol Endocrinol. 2007;21:457–471. doi: 10.1210/me.2005-0216. [DOI] [PubMed] [Google Scholar]
- Kim HH, Wolfe A, Smith GR, Tobet SA, Radovick S. Promoter sequences targeting tissue-specific gene expression of hypothalamic and ovarian gonadotropin-releasing hormone in vivo. J Biol Chem. 2002;277:5194–5202. doi: 10.1074/jbc.M110535200. [DOI] [PubMed] [Google Scholar]
- Kim KH, Patel L, Tobet SA, King JC, Rubin BS, Stopa EG. Gonadotropin-releasing hormone immunoreactivity in the adult and fetal human olfactory system. Brain Res. 1999;826:220–229. doi: 10.1016/s0006-8993(99)01271-8. [DOI] [PubMed] [Google Scholar]
- King JC, Rubin BS. GnRH Subgroups: A Microarchitecture. In: Crowley WF Jr, Conn PM, editors. Modes of Action of GnRH and GnRH Analogs. New York: Springer-Verlag; 1992. pp. 161–178. [Google Scholar]
- King JC, Tobet SA, Snavely FL, Arimura AA. LHRH Imminopositive Cells and Their Projections to the Median Eminence and Organum Vasculosum of the Lamina Teminalis. J Comp Neurol. 1982;209:287–300. doi: 10.1002/cne.902090307. [DOI] [PubMed] [Google Scholar]
- Kirilov M, Clarkson J, Liu X, Roa J, Campos P, Porteous R, Schutz G, Herbison AE. Dependence of fertility on kisspeptin-Gpr54 signaling at the GnRH neuron. Nat Commun. 2013;4:2492. doi: 10.1038/ncomms3492. [DOI] [PubMed] [Google Scholar]
- Kjeldsen T, Andersen AS, Wiberg FC, Rasmussen JS, Schaffer L, Balschmidt P, Moller KB, Moller NP. The ligand specificities of the insulin receptor and the insulin-like growth factor I receptor reside in different regions of a common binding site. Proc Natl Acad Sci U S A. 1991;88:4404–4408. doi: 10.1073/pnas.88.10.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner J, Savontaus E, Chua SC, Jr, Leibel RL, Wardlaw SL. Leptin regulation of Agrp and Npy mRNA in the rat hypothalamus. J Neuroendocrinol. 2001;13:959–966. doi: 10.1046/j.1365-2826.2001.00716.x. [DOI] [PubMed] [Google Scholar]
- Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- Kovacs P, Parlow AF, Karkanias GB. Effect of centrally administered insulin on gonadotropin-releasing hormone neuron activity and luteinizing hormone surge in the diabetic female rat. Neuroendocrinology. 2002;76:357–365. doi: 10.1159/000067585. [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Dominguez CE, Yen SS. Nutritional and endocrine-metabolic aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 1998;83:25–32. doi: 10.1210/jcem.83.1.4502. [DOI] [PubMed] [Google Scholar]
- Lawson MA, Buhain AR, Jovenal JC, Mellon PL. Multiple factors interacting at the GATA sites of the gonadotropin- releasing hormone neuron-specific enhancer regulate gene expression. Mol Endocrinol. 1998;12:364–377. doi: 10.1210/mend.12.3.0082. [DOI] [PubMed] [Google Scholar]
- Lawson MA, Jain S, Sun S, Patel K, Malcolm PJ, Chang RJ. Evidence for insulin suppression of baseline luteinizing hormone in women with polycystic ovarian syndrome and normal women. J Clin Endocrinol Metab. 2008;93:2089–2096. doi: 10.1210/jc.2007-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrethon MC, Vandersmissen E, Gerard A, Parent AS, Bourguignon JP. Cocaine and amphetamine-regulated-transcript peptide mediation of leptin stimulatory effect on the rat gonadotropin-releasing hormone pulse generator in vitro. J Neuroendocrinol. 2000;12:383–385. doi: 10.1046/j.1365-2826.2000.00497.x. [DOI] [PubMed] [Google Scholar]
- Lee K, Porteous R, Campbell RE, Luscher B, Herbison AE. Knockdown of GABA(A) receptor signaling in GnRH neurons has minimal effects upon fertility. Endocrinology. 2010;151:4428–4436. doi: 10.1210/en.2010-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legan SJ, Karsch FJ. A daily signal for the LH surge in the rat. Endocrinology. 1975;96:57–62. doi: 10.1210/endo-96-1-57. [DOI] [PubMed] [Google Scholar]
- LeRoith D, Adamo M, Shemer J, Waldbillig R, Lesniak MA, dePablo F, Hart C, Roth J. Insulin-related materials in the nervous system of vertebrates and non-vertebrates: possible extrapancreatic production. Horm Metab Res. 1988;20:411–420. doi: 10.1055/s-2007-1010850. [DOI] [PubMed] [Google Scholar]
- LeRoith D, Kavsan VM, Koval AP, Roberts CT., Jr Phylogeny of the insulin-like growth factors (IGFs) and receptors: a molecular approach. Mol Reprod Dev. 1993;35:332–6. doi: 10.1002/mrd.1080350403. discussion 337–8. [DOI] [PubMed] [Google Scholar]
- Leslie RA, Sanders SJ, Anderson SI, Schuhler S, Horan TL, Ebling FJ. Appositions between cocaine and amphetamine-related transcript- and gonadotropin releasing hormone-immunoreactive neurons in the hypothalamus of the Siberian hamster. Neurosci Lett. 2001;314:111–114. doi: 10.1016/s0304-3940(01)02291-1. [DOI] [PubMed] [Google Scholar]
- Lima FB, Centeno ML, Costa ME, Reddy AP, Cameron JL, Bethea CL. Stress sensitive female macaques have decreased fifth Ewing variant (Fev) and serotonin-related gene expression that is not reversed by citalopram. Neuroscience. 2009;164:676–691. doi: 10.1016/j.neuroscience.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- Lomniczi A, Loche A, Castellano JM, Ronnekleiv OK, Bosch M, Kaidar G, Knoll JG, Wright H, Pfeifer GP, Ojeda SR. Epigenetic control of female puberty. Nat Neurosci. 2013;16:281–289. doi: 10.1038/nn.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long DJ, 2nd, Buggs C. Microwave oven-based technique for immunofluorescent staining of paraffin-embedded tissues. J Mol Histol. 2008;39:1–4. doi: 10.1007/s10735-007-9093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo KM, Sun Y, Gore AC. Insulin-like growth factor-I effects on gonadotropin-releasing hormone biosynthesis in GT1-7 cells. Endocrinology. 1998;139:1125–1132. doi: 10.1210/endo.139.3.5852. [DOI] [PubMed] [Google Scholar]
- Lu M, Tang Q, Olefsky JM, Mellon PL, Webster NJ. Adiponectin activates adenosine monophosphate-activated protein kinase and decreases luteinizing hormone secretion in LbetaT2 gonadotropes. Mol Endocrinol. 2008;22:760–771. doi: 10.1210/me.2007-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna AM, Wilson DM, Wibbelsman CJ, Brown RC, Nagashima RJ, Hintz RL, Rosenfeld RG. Somatomedins in adolescence: a cross-sectional study of the effect of puberty on plasma insulin-like growth factor I and II levels. J Clin Endocrinol Metab. 1983;57:268–271. doi: 10.1210/jcem-57-2-268. [DOI] [PubMed] [Google Scholar]
- Ma X, Dong Y, Matzuk MM, Kumar TR. Targeted disruption of luteinizing hormone beta-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci U S A. 2004;101:17294–17299. doi: 10.1073/pnas.0404743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Baxter RC. Insulin-like growth factor binding protein-3: biochemistry and physiology. Growth Regul. 1992;2:88–99. [PubMed] [Google Scholar]
- Mason AJ, Hayflick JS, Zoeller RT, Young WS, Phillips HS, Nikolics K, Seeburg PH. A deletion truncating the gonadotropin-releasing hormone gene is responsible for hypogonadism in the hpg mouse. Science. 1986;234:1366–1371. doi: 10.1126/science.3024317. [DOI] [PubMed] [Google Scholar]
- Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320:383–388. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, Levine JE. Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proc Natl Acad Sci U S A. 2010;107:22693–22698. doi: 10.1073/pnas.1012406108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer C, Boehm U. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci. 2011;14:704–710. doi: 10.1038/nn.2818. [DOI] [PubMed] [Google Scholar]
- McRory JE, Sherwood NM. Ancient divergence of insulin and insulin-like growth factor. DNA Cell Biol. 1997;16:939–949. doi: 10.1089/dna.1997.16.939. [DOI] [PubMed] [Google Scholar]
- Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI. Immortalization of Hypothalamic GnRH Neurons by Genetically Targeted Tumorigenesis. Neuron. 1990;5:1–10. doi: 10.1016/0896-6273(90)90028-e. [DOI] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin-releasing hormone receptors. Endocr Rev. 2004;25:235–275. doi: 10.1210/er.2003-0002. [DOI] [PubMed] [Google Scholar]
- Miller BH, Gore AC. Alterations in hypothalamic insulin-like growth factor-I and its associations with gonadotropin releasing hormone neurones during reproductive development and ageing. J Neuroendocrinol. 2001;13:728–736. doi: 10.1046/j.1365-2826.2001.00686.x. [DOI] [PubMed] [Google Scholar]
- Miller DW, Blache D, Boukhliq R, Curlewis JD, Martin GB. Central metabolic messengers and the effects of nutrition on gonadotrophin secretion in sheep. J Reprod Fertil. 1998;112:347–356. doi: 10.1530/jrf.0.1120347. [DOI] [PubMed] [Google Scholar]
- Miller DW, Blache D, Martin GB. The role of intracerebral insulin in the effect of nutrition on gonadotrophin secretion in mature male sheep. J Endocrinol. 1995;147:321–329. doi: 10.1677/joe.0.1470321. [DOI] [PubMed] [Google Scholar]
- Miller RS, Wolfe A, He L, Radovick S, Wondisford FE. CREB binding protein (CBP) activation is required for luteinizing hormone beta expression and normal fertility in mice. Mol Cell Biol. 2012;32:2349–2358. doi: 10.1128/MCB.00394-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M, Armstrong DT, Robker RL, Norman RJ. Adipokines: implications for female fertility and obesity. Reproduction. 2005;130:583–597. doi: 10.1530/rep.1.00521. [DOI] [PubMed] [Google Scholar]
- Modric T, Silha JV, Shi Z, Gui Y, Suwanichkul A, Durham SK, Powell DR, Murphy LJ. Phenotypic manifestations of insulin-like growth factor-binding protein-3 overexpression in transgenic mice. Endocrinology. 2001;142:1958–1967. doi: 10.1210/endo.142.5.8165. [DOI] [PubMed] [Google Scholar]
- Moret M, Stettler R, Rodieux F, Gaillard RC, Waeber G, Wirthner D, Giusti V, Tappy L, Pralong FP. Insulin modulation of luteinizing hormone secretion in normal female volunteers and lean polycystic ovary syndrome patients. Neuroendocrinology. 2009;89:131–139. doi: 10.1159/000160911. [DOI] [PubMed] [Google Scholar]
- Mounzih K, Lu R, Chehab FF. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology. 1997;138:1190–1193. doi: 10.1210/endo.138.3.5024. [DOI] [PubMed] [Google Scholar]
- Moxham CP, Duronio V, Jacobs S. Insulin-like growth factor I receptor beta-subunit heterogeneity. Evidence for hybrid tetramers composed of insulin-like growth factor I and insulin receptor heterodimers. J Biol Chem. 1989;264:13238–13244. [PubMed] [Google Scholar]
- Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- Murahashi K, Bucholtz DC, Nagatani S, Tsukahara S, Tsukamura H, Foster DL, Maeda KI. Suppression of luteinizing hormone pulses by restriction of glucose availability is mediated by sensors in the brain stem. Endocrinology. 1996;137:1171–1176. doi: 10.1210/endo.137.4.8625886. [DOI] [PubMed] [Google Scholar]
- Mutiara S, Kanasaki H, Harada T, Oride A, Miyazaki K. The involvement of phosphatidylinositol 3-kinase in gonadotropin-releasing hormone-induced gonadotropin alpha- and FSHbeta-subunit genes expression in clonal gonadotroph LbetaT2 cells. Mol Cell Endocrinol. 2008;283:1–11. doi: 10.1016/j.mce.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Nakae J, Biggs WH, 3rd, Kitamura T, Cavenee WK, Wright CV, Arden KC, Accili D. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet. 2002;32:245–253. doi: 10.1038/ng890. [DOI] [PubMed] [Google Scholar]
- Nakae J, Kido Y, Accili D. Distinct and overlapping functions of insulin and IGF-I receptors. Endocr Rev. 2001;22:818–835. doi: 10.1210/edrv.22.6.0452. [DOI] [PubMed] [Google Scholar]
- Nam SY, Lee EJ, Kim KR, Cha BS, Song YD, Lim SK, Lee HC, Huh KB. Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int J Obes Relat Metab Disord. 1997;21:355–359. doi: 10.1038/sj.ijo.0800412. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Barreiro ML, Casanueva FF, Aguilar E, Dieguez C, et al. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology. 2005;146:1689–1697. doi: 10.1210/en.2004-1353. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, et al. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004;561:379–386. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Kaiser UB. Metabolic influences on neuroendocrine regulation of reproduction. Curr Opin Endocrinol Diabetes Obes. 2013;20:335–341. doi: 10.1097/MED.0b013e32836318ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratil AM, Song H, Hernandez JB, Cherrington BD, Santos SJ, Low JM, Do MH, Lawson MA. Insulin augments gonadotropin-releasing hormone induction of translation in LbetaT2 cells. Mol Cell Endocrinol. 2009;311:47–54. doi: 10.1016/j.mce.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning Y, Schuller AG, Bradshaw S, Rotwein P, Ludwig T, Frystyk J, Pintar JE. Diminished growth and enhanced glucose metabolism in triple knockout mice containing mutations of insulin-like growth factor binding protein-3, -4, and -5. Mol Endocrinol. 2006;20:2173–2186. doi: 10.1210/me.2005-0196. [DOI] [PubMed] [Google Scholar]
- Novaira HJ, Sonko ML, Hoffman G, Koo Y, Ko C, Wolfe A, Radovick S. Disrupted kisspeptin signaling in GnRH neurons leads to reproductive abnormalities and HH. Molecular Endocrinology. 2013 doi: 10.1210/me.2013-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novaira HJ, Fadoju D, Diaczok D, Radovick S. Genetic mechanisms mediating kisspeptin regulation of GnRH gene expression. J Neurosci. 2012;32:17391–17400. doi: 10.1523/JNEUROSCI.2438-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novaira HJ, Yates M, Diaczok D, Kim H, Wolfe A, Radovick S. The gonadotropin-releasing hormone cell-specific element is required for normal puberty and estrous cyclicity. J Neurosci. 2011;31:3336–3343. doi: 10.1523/JNEUROSCI.5419-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- Okamura H, Tsukamura H, Ohkura S, Uenoyama Y, Wakabayashi Y, Maeda K. Kisspeptin and GnRH pulse generation. Adv Exp Med Biol. 2013;784:297–323. doi: 10.1007/978-1-4614-6199-9_14. [DOI] [PubMed] [Google Scholar]
- Olson BR, Scott DC, Wetsel WC, Elliot SJ, Tomic M, Stojilkovic S, Nieman LK, Wray S. Effects of insulin-like growth factors I and II and insulin on the immortalized hypothalamic GTI-7 cell line. Neuroendocrinology. 1995;62:155–165. doi: 10.1159/000127000. [DOI] [PubMed] [Google Scholar]
- Olster DH, Foster DL. Control of gonadotropin secretion in the male during puberty: a decrease in response to steroid inhibitory feedback in the absence of an increase in steroid-independent drive in the sheep. Endocrinology. 1986;118:2225–2234. doi: 10.1210/endo-118-6-2225. [DOI] [PubMed] [Google Scholar]
- Oltmanns KM, Fruehwald-Schultes B, Kern W, Born J, Fehm HL, Peters A. Hypoglycemia, but not insulin, acutely decreases LH and T secretion in men. J Clin Endocrinol Metab. 2001;86:4913–4919. doi: 10.1210/jcem.86.10.7892. [DOI] [PubMed] [Google Scholar]
- Ooi GT, Tawadros N, Escalona RM. Pituitary cell lines and their endocrine applications. Mol Cell Endocrinol. 2004;228:1–21. doi: 10.1016/j.mce.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Pape JR, Skynner MJ, Allen ND, Herbison AE. Transgenics identify distal 5′- and 3′-sequences specifying gonadotropin-releasing hormone expression in adult mice. Mol Endocrinol. 1999;13:2203–2211. doi: 10.1210/mend.13.12.0380. [DOI] [PubMed] [Google Scholar]
- Parent AS, Lebrethon MC, Gerard A, Vandersmissen E, Bourguignon JP. Leptin effects on pulsatile gonadotropin releasing hormone secretion from the adult rat hypothalamus and interaction with cocaine and amphetamine regulated transcript peptide and neuropeptide Y. Regul Pept. 2000;92:17–24. doi: 10.1016/s0167-0115(00)00144-0. [DOI] [PubMed] [Google Scholar]
- Parhar IS, Ogawa S, Sakuma Y. Laser-captured single digoxigenin-labeled neurons of gonadotropin-releasing hormone types reveal a novel G protein-coupled receptor (Gpr54) during maturation in cichlid fish. Endocrinology. 2004;145:3613–3618. doi: 10.1210/en.2004-0395. [DOI] [PubMed] [Google Scholar]
- Payne AH. Hormonal regulation of cytochrome P450 enzymes, cholesterol side-chain cleavage and 17 alpha-hydroxylase/C17-20 lyase in Leydig cells. Biol Reprod. 1990;42:399–404. doi: 10.1095/biolreprod42.3.399. [DOI] [PubMed] [Google Scholar]
- Pazos F, Sanchez-Franco F, Balsa J, Lopez-Fernandez J, Escalada J, Cacicedo L. Regulation of gonadal and somatotropic axis by chronic intraventricular infusion of insulin-like growth factor 1 antibody at the initiation of puberty in male rats. Neuroendocrinology. 1999;69:408–416. doi: 10.1159/000054443. [DOI] [PubMed] [Google Scholar]
- Pesant MH, Dwyer A, Marques Vidal P, Schneiter P, Giusti V, Tappy L, Pralong FP. The lack of effect of insulin on luteinizing hormone pulsatility in healthy male volunteers provides evidence of a sexual dimorphism in the metabolic regulation of reproductive hormones. Am J Clin Nutr. 2012;96:283–288. doi: 10.3945/ajcn.111.030189. [DOI] [PubMed] [Google Scholar]
- Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- Plant TM, Barker-Gibb ML. Neurobiological mechanisms of puberty in higher primates. Hum Reprod Update. 2004;10:67–77. doi: 10.1093/humupd/dmh001. [DOI] [PubMed] [Google Scholar]
- Pohl CR, deRidder CM, Plant TM. Gonadal and nongonadal mechanisms contribute to the prepubertal hiatus in gonadotropin secretion in the female rhesus monkey (Macaca mulatta) J Clin Endocrinol Metab. 1995;80:2094–2101. doi: 10.1210/jcem.80.7.7608261. [DOI] [PubMed] [Google Scholar]
- Popa SM, Moriyama RM, Caligioni CS, Yang JJ, Cho CM, Concepcion TL, Oakley AE, Lee IH, Sanz E, Amieux PS, et al. Redundancy in Kiss1 expression safeguards reproduction in the mouse. Endocrinology. 2013;154:2784–2794. doi: 10.1210/en.2013-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popolow HB, King JC, Gerall AA. Rostral medial preoptic area lesions’ influence on female estrous processes and LHRH distribution. Physiol Behav. 1981;27:855–861. doi: 10.1016/0031-9384(81)90053-6. [DOI] [PubMed] [Google Scholar]
- Powolny AA, Wang S, Carlton PS, Hoot DR, Clinton SK. Interrelationships between dietary restriction, the IGF-I axis, and expression of vascular endothelial growth factor by prostate adenocarcinoma in rats. Mol Carcinog. 2008;47:458–465. doi: 10.1002/mc.20403. [DOI] [PubMed] [Google Scholar]
- Qiu X, Dowling AR, Marino JS, Faulkner LD, Bryant B, Bruning JC, Elias CF, Hill JW. Delayed puberty but normal fertility in mice with selective deletion of insulin receptors from Kiss1 cells. Endocrinology. 2013;154:1337–1348. doi: 10.1210/en.2012-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quennell JH, Howell CS, Roa J, Augustine RA, Grattan DR, Anderson GM. Leptin deficiency and diet-induced obesity reduce hypothalamic kisspeptin expression in mice. Endocrinology. 2011;152:1541–1550. doi: 10.1210/en.2010-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quennell JH, Mulligan AC, Tups A, Liu X, Phipps SJ, Kemp CJ, Herbison AE, Grattan DR, Anderson GM. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology. 2009;150:2805–2812. doi: 10.1210/en.2008-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada A, Etgen AM. Functional interactions between estrogen and insulin-like growth factor-I in the regulation of alpha 1B-adrenoceptors and female reproductive function. J Neurosci. 2002;22:2401–2408. doi: 10.1523/JNEUROSCI.22-06-02401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radovick S, Wondisford FE, Nakayama Y, Yamada M, Cutler GB, Jr, Weintraub BD. Isolation and Characterization of the Human Gonadotropin- Releasing Hormone Gene in the Hypothalamus and Placenta. Mol Endo. 1990;4:476–480. doi: 10.1210/mend-4-3-476. [DOI] [PubMed] [Google Scholar]
- Radovick S, Wray S, Lee E, Nicols DK, Nakayama Y, Weintraub BD, Westphal H, Cutler GB, Jr, Wondisford FE. Migratory arrest of gonadotropin-releasing hormone neurons in transgenic mice. Proc Natl Acad Sci USA. 1991;88:3402–3406. doi: 10.1073/pnas.88.8.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rispoli LA, Nett TM. Pituitary gonadotropin-releasing hormone (GnRH) receptor: structure, distribution and regulation of expression. Anim Reprod Sci. 2005;88:57–74. doi: 10.1016/j.anireprosci.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Rivier C. Neuroendocrine effects of cytokines in the rat. Rev Neurosci. 1993;4:223–237. doi: 10.1515/revneuro.1993.4.3.223. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Arias P, Refojo D, Feleder C, Moguilevsky J. Arrest of pulsatile luteinizing hormone (LH) secretion during insulin- induced hypoglycemia (IIH): improvement by intrahypothalamic perfusion with glucose. Exp Clin Endocrinol Diabetes. 1999;107:257–261. doi: 10.1055/s-0029-1212109. [DOI] [PubMed] [Google Scholar]
- Rodriguez SS, Schwerdt JI, Barbeito CG, Flamini MA, Han Y, Bohn MC, Goya RG. Hypothalamic IGF-I gene therapy prolongs estrous cyclicity and protects ovarian structure in middle-aged female rats. Endocrinology. 2013;154:2166–2173. doi: 10.1210/en.2013-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland AV, Moenter SM. Glucosensing by GnRH neurons: inhibition by androgens and involvement of AMP-activated protein kinase. Mol Endocrinol. 2011a;25:847–858. doi: 10.1210/me.2010-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland AV, Moenter SM. Regulation of gonadotropin-releasing hormone neurons by glucose. Trends Endocrinol Metab. 2011b;22:443–449. doi: 10.1016/j.tem.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose A, Froment P, Perrot V, Quon MJ, LeRoith D, Dupont J. The luteinizing hormone-releasing hormone inhibits the anti-apoptotic activity of insulin-like growth factor-1 in pituitary alphaT3 cells by protein kinase Calpha-mediated negative regulation of Akt. J Biol Chem. 2004;279:52500–52516. doi: 10.1074/jbc.M404571200. [DOI] [PubMed] [Google Scholar]
- Ross JL, Loriaux DL, Cutler GB., Jr Developmental changes in neuroendocrine regulation of gonadotropin secretion in gonadal dysgenesis. J Clin Endocrinol Metab. 1983;57:288–293. doi: 10.1210/jcem-57-2-288. [DOI] [PubMed] [Google Scholar]
- Ryan KJ. Granulosa-thecal cell interaction in ovarian steroidogenesis. J Steroid Biochem. 1979;11:799–800. doi: 10.1016/0022-4731(79)90014-1. [DOI] [PubMed] [Google Scholar]
- Sakai K, Clemmons DR. Glucosamine induces resistance to insulin-like growth factor I (IGF-I) and insulin in Hep G2 cell cultures: biological significance of IGF-I/insulin hybrid receptors. Endocrinology. 2003;144:2388–2395. doi: 10.1210/en.2002-221133. [DOI] [PubMed] [Google Scholar]
- Salerno M, Argenziano A, Di Maio S, Gasparini N, Formicola S, De Filippo G, Tenore A. Pubertal growth, sexual maturation, and final height in children with IDDM. Effects of age at onset and metabolic control. Diabetes Care. 1997;20:721–724. doi: 10.2337/diacare.20.5.721. [DOI] [PubMed] [Google Scholar]
- Salih DA, Tripathi G, Holding C, Szestak TA, Gonzalez MI, Carter EJ, Cobb LJ, Eisemann JE, Pell JM. Insulin-like growth factor-binding protein 5 (Igfbp5) compromises survival, growth, muscle development, and fertility in mice. Proc Natl Acad Sci U S A. 2004;101:4314–4319. doi: 10.1073/pnas.0400230101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi R, Castillo E, Voirol MJ, Glauser M, Rey JP, Gaillard RC, Vollenweider P, Pralong FP. Gonadotropin-releasing hormone-expressing neurons immortalized conditionally are activated by insulin: implication of the mitogen-activated protein kinase pathway. Endocrinology. 2006;147:816–826. doi: 10.1210/en.2005-0728. [DOI] [PubMed] [Google Scholar]
- Schneider JE, Wade GN. Decreased availability of metabolic fuels induces anestrus in golden hamsters. Am J Physiol. 1990;258:R750–R755. doi: 10.1152/ajpregu.1990.258.3.R750. [DOI] [PubMed] [Google Scholar]
- Schneider JE, Wade GN. Availability of metabolic fuels controls estrous cyclicity of Syrian hamsters. Science. 1989;244:1326–1328. doi: 10.1126/science.2734610. [DOI] [PubMed] [Google Scholar]
- Schreihofer DA, Amico JA, Cameron JL. Reversal of fasting-induced suppression of luteinizing hormone (LH) secretion in male rhesus monkeys by intragastric nutrient infusion: evidence for rapid stimulation of LH by nutritional signals. Endocrinology. 1993a;132:1890–1897. doi: 10.1210/endo.132.5.8477642. [DOI] [PubMed] [Google Scholar]
- Schreihofer DA, Parfitt DB, Cameron JL. Suppression of luteinizing hormone secretion during short-term fasting in male rhesus monkeys: the role of metabolic versus stress signals. Endocrinology. 1993b;132:1881–1889. doi: 10.1210/endo.132.5.8477641. [DOI] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;338:161–164. doi: 10.1038/338161a0. [DOI] [PubMed] [Google Scholar]
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Senashova O, Reddy AP, Cameron JL, Bethea CL. The effect of citalopram on midbrain CRF receptors 1 and 2 in a primate model of stress-induced amenorrhea. Reprod Sci. 2012;19:623–632. doi: 10.1177/1933719111430992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- Shimshek DR, Bus T, Grinevich V, Single FN, Mack V, Sprengel R, Spergel DJ, Seeburg PH. Impaired reproductive behavior by lack of GluR-B containing AMPA receptors but not of NMDA receptors in hypothalamic and septal neurons. Mol Endocrinol. 2006;20:219–231. doi: 10.1210/me.2005-0262. [DOI] [PubMed] [Google Scholar]
- Shimshek DR, Kim J, Hubner MR, Spergel DJ, Buchholz F, Casanova E, Stewart AF, Seeburg PH, Sprengel R. Codon-improved Cre recombinase (iCre) expression in the mouse. Genesis. 2002;32:19–26. doi: 10.1002/gene.10023. [DOI] [PubMed] [Google Scholar]
- Singh SP, Wolfe A, Ng Y, DiVall SA, Buggs C, Levine JE, Wondisford FE, Radovick S. Impaired estrogen feedback and infertility in female mice with pituitary-specific deletion of estrogen receptor alpha (ESR1) Biol Reprod. 2009;81:488–496. doi: 10.1095/biolreprod.108.075259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skynner MJ, Slater R, Sim JA, Allen ND, Herbison AE. Promoter transgenics reveal multiple gonadotropin-releasing hormone-I-expressing cell populations of different embryological origin in mouse brain. J Neurosci. 1999;19:5955–5966. doi: 10.1523/JNEUROSCI.19-14-05955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slyper AH. The pubertal timing controversy in the USA, and a review of possible causative factors for the advance in timing of onset of puberty. Clin Endocrinol (Oxf) 2006;65:1–8. doi: 10.1111/j.1365-2265.2006.02539.x. [DOI] [PubMed] [Google Scholar]
- Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005a;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005b;146:2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- Soldani R, Cagnacci A, Paoletti AM, Yen SS, Melis GB. Modulation of anterior pituitary luteinizing hormone response to gonadotropin-releasing hormone by insulin-like growth factor I in vitro. Fertil Steril. 1995;64:634–637. doi: 10.1016/s0015-0282(16)57804-2. [DOI] [PubMed] [Google Scholar]
- Soldani R, Cagnacci A, Yen SS. Insulin, insulin-like growth factor I (IGF-I) and IGF-II enhance basal and gonadotrophin-releasing hormone-stimulated luteinizing hormone release from rat anterior pituitary cells in vitro. Eur J Endocrinol. 1994;131:641–645. doi: 10.1530/eje.0.1310641. [DOI] [PubMed] [Google Scholar]
- Soos MA, Siddle K. Immunological relationships between receptors for insulin and insulin-like growth factor I. Evidence for structural heterogeneity of insulin-like growth factor I receptors involving hybrids with insulin receptors. Biochem J. 1989;263:553–563. doi: 10.1042/bj2630553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislaus D, Pinter JH, Janovick JA, Conn PM. Mechanisms mediating multiple physiological responses to gonadotropin-releasing hormone. Mol Cell Endocrinol. 1998a;144:1–10. doi: 10.1016/s0303-7207(98)00126-9. [DOI] [PubMed] [Google Scholar]
- Stanislaus D, Ponder S, Ji TH, Conn PM. Gonadotropin-releasing hormone receptor couples to multiple G proteins in rat gonadotrophs and in GGH3 cells: evidence from palmitoylation and overexpression of G proteins. Biol Reprod. 1998b;59:579–586. doi: 10.1095/biolreprod59.3.579. [DOI] [PubMed] [Google Scholar]
- Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev. 2007;28:117–149. doi: 10.1210/er.2006-0022. [DOI] [PubMed] [Google Scholar]
- Stoving RK, Flyvbjerg A, Frystyk J, Fisker S, Hangaard J, Hansen-Nord M, Hagen C. Low serum levels of free and total insulin-like growth factor I (IGF-I) in patients with anorexia nervosa are not associated with increased IGF-binding protein-3 proteolysis. J Clin Endocrinol Metab. 1999;84:1346–1350. doi: 10.1210/jcem.84.4.5622. [DOI] [PubMed] [Google Scholar]
- Sun Y, Todd BJ, Thornton K, Etgen AM, Neal-Perry G. Differential effects of hypothalamic IGF-I on gonadotropin releasing hormone neuronal activation during steroid-induced LH surges in young and middle-aged female rats. Endocrinology. 2011;152:4276–4287. doi: 10.1210/en.2011-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter KJ, Pohl CR, Plant TM. Indirect assessment of pulsatile gonadotropin-releasing hormone release in agonadal prepubertal rhesus monkeys (Macaca mulatta) J Endocrinol. 1999;160:35–41. doi: 10.1677/joe.0.1600035. [DOI] [PubMed] [Google Scholar]
- Suter KJ, Pohl CR, Wilson ME. Circulating concentrations of nocturnal leptin, growth hormone, and insulin-like growth factor-I increase before the onset of puberty in agonadal male monkeys: potential signals for the initiation of puberty. J Clin Endocrinol Metab. 2000;85:808–814. doi: 10.1210/jcem.85.2.6371. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Nagatani S, Bucholtz DC, Ohkura S, Tsukamura H, Maeda K, Foster DL. Central action of insulin regulates pulsatile luteinizing hormone secretion in the diabetic sheep model. Biol Reprod. 2000;62:1256–1261. doi: 10.1095/biolreprod62.5.1256. [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M. Ghrelin and reproduction: ghrelin as novel regulator of the gonadotropic axis. Vitam Horm. 2008;77:285–300. doi: 10.1016/S0083-6729(06)77012-1. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Bridson WE, Nass TE, Noonan JJ, Dierschke DJ. Developmental changes in the luteinizing hormone secretory pattern in peripubertal female rhesus monkeys: comparisons between gonadally intact and ovariectomized animals. Endocrinology. 1984;115:2233–2240. doi: 10.1210/endo-115-6-2233. [DOI] [PubMed] [Google Scholar]
- Todd BJ, Merhi ZO, Shu J, Etgen AM, Neal-Perry GS. Hypothalamic insulin-like growth factor-I receptors are necessary for hormone-dependent luteinizing hormone surges: implications for female reproductive aging. Endocrinology. 2010;151:1356–1366. doi: 10.1210/en.2009-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomikawa J, Uenoyama Y, Ozawa M, Fukanuma T, Takase K, Goto T, Abe H, Ieda N, Minabe S, Deura C, et al. Epigenetic regulation of Kiss1 gene expression mediating estrogen-positive feedback action in the mouse brain. Proc Natl Acad Sci U S A. 2012;109:E1294–301. doi: 10.1073/pnas.1114245109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortoriello DV, McMinn J, Chua SC. Dietary-induced obesity and hypothalamic infertility in female DBA/2J mice. Endocrinology. 2004;145:1238–1247. doi: 10.1210/en.2003-1406. [DOI] [PubMed] [Google Scholar]
- Tortoriello DV, McMinn JE, Chua SC. Increased expression of hypothalamic leptin receptor and adiponectin accompany resistance to dietary-induced obesity and infertility in female C57BL/6J mice. Int J Obes (Lond) 2007;31:395–402. doi: 10.1038/sj.ijo.0803392. [DOI] [PubMed] [Google Scholar]
- Tran S, Zhou X, Lafleur C, Calderon MJ, Ellsworth BS, Kimmins S, Boehm U, Treier M, Boerboom D, Bernard DJ. Impaired fertility and FSH synthesis in gonadotrope-specific Foxl2 knockout mice. Mol Endocrinol. 2013;27:407–421. doi: 10.1210/me.2012-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway JL, Morrison BD, Goldfine ID, Pessin JE. Assembly of insulin/insulin-like growth factor-1 hybrid receptors in vitro. J Biol Chem. 1989;264:21450–21453. [PubMed] [Google Scholar]
- Tsutsumi M, Zhou W, Millar RP, Mellon PL, Roberts JL, Flanagan CA, Dong K, Gillo B, Sealfon SC. Cloning and functional expression of a mouse gonadotropin-releasing hormone receptor. Mol Endocrinol. 1992;6:1163–1169. doi: 10.1210/mend.6.7.1324422. [DOI] [PubMed] [Google Scholar]
- Unger JW, Lange W. Insulin receptors in the pituitary gland: morphological evidence for influence on opioid peptide-synthesizing cells. Cell Tissue Res. 1997;288:471–483. doi: 10.1007/s004410050833. [DOI] [PubMed] [Google Scholar]
- Van Goor F, Krsmanovic LZ, Catt KJ, Stojilkovic SS. Autocrine regulation of calcium influx and gonadotropin-releasing hormone secretion in hypothalamic neurons. Biochem Cell Biol. 2000;78:359–370. [PubMed] [Google Scholar]
- van Houten M, Posner BI, Kopriwa BM, Brawer JR. Insulin binding sites localized to nerve terminals in rat median eminence and arcuate nucleus. Science. 1980;207:1081–1083. doi: 10.1126/science.6986652. [DOI] [PubMed] [Google Scholar]
- Vulliemoz NR, Xiao E, Xia-Zhang L, Wardlaw SL, Ferin M. Central infusion of agouti-related peptide suppresses pulsatile luteinizing hormone release in the ovariectomized rhesus monkey. Endocrinology. 2005;146:784–789. doi: 10.1210/en.2004-1093. [DOI] [PubMed] [Google Scholar]
- Wade GN, Jones JE. Neuroendocrinology of nutritional infertility. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1277–96. doi: 10.1152/ajpregu.00475.2004. [DOI] [PubMed] [Google Scholar]
- Wahab F, Ullah F, Chan YM, Seminara SB, Shahab M. Decrease in hypothalamic Kiss1 and Kiss1r expression: a potential mechanism for fasting-induced suppression of the HPG axis in the adult male rhesus monkey (Macaca mulatta) Horm Metab Res. 2011;43:81–85. doi: 10.1055/s-0030-1269852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi Y, Yamamura T, Sakamoto K, Mori Y, Okamura H. Electrophysiological and morphological evidence for synchronized GnRH pulse generator activity among Kisspeptin/neurokinin B/dynorphin A (KNDy) neurons in goats. J Reprod Dev. 2013;59:40–48. doi: 10.1262/jrd.2012-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JM, Chi M, Polack S, Diedrich K, Ortmann O. Interactions of insulin-like growth factors and estradiol in rat pituitary gonadotrophs. Growth Factors. 2003;21:61–69. doi: 10.1080/08977190310001605760. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Xia YX, Polack S, Diedrich K, Ortmann O. Short-term effects of IGF-I and estradiol on LH secretion from female rat gonadotrophs. Growth Horm IGF Res. 2006;16:357–364. doi: 10.1016/j.ghir.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Wen S, Schwarz JR, Niculescu D, Dinu C, Bauer CK, Hirdes W, Boehm U. Functional characterization of genetically labeled gonadotropes. Endocrinology. 2008;149:2701–2711. doi: 10.1210/en.2007-1502. [DOI] [PubMed] [Google Scholar]
- Whirledge S, Cidlowski JA. Glucocorticoids, stress, and fertility. Minerva Endocrinol. 2010;35:109–125. [PMC free article] [PubMed] [Google Scholar]
- Whitley NC, Barb CR, Utley RV, Popwell JM, Kraeling RR, Rampacek GB. Influence of stage of the estrous cycle on insulin-like growth factor-I modulation of luteinizing hormone secretion in the gilt. Biol Reprod. 1995;53:1359–1364. doi: 10.1095/biolreprod53.6.1359. [DOI] [PubMed] [Google Scholar]
- Whyte DB, Lawson MA, Belsham DD, Eraly SA, Bond CT, Adelman JP, Mellon PL. A neuron-specific enhancer targets expression of the gonadotropin-releasing hormone gene to hypothalamic neurosecretory neurons. Mol Endo. 1995;9:467–477. doi: 10.1210/mend.9.4.7659090. [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982;34:395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E, Bridson WE, Goy RW. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology. 1980;31:147–157. doi: 10.1159/000123066. [DOI] [PubMed] [Google Scholar]
- Wierman ME, Xu M, Pierce A, Bliesner B, Bliss SP, Roberson MS. Extracellular signal-regulated kinase 1 and 2 are not required for GnRH neuron development and normal female reproductive axis function in mice. Neuroendocrinology. 2012;95:289–296. doi: 10.1159/000331389. [DOI] [PubMed] [Google Scholar]
- Wilson PW, Umpierrez GE. Insulin resistance and pubertal changes. J Clin Endocrinol Metab. 2008;93:2472–2473. doi: 10.1210/jc.2008-0873. [DOI] [PubMed] [Google Scholar]
- Windle JJ, Weiner RI, Mellon PL. Cell lines of the pituitary gonadotrope lineage derived by targeted oncogenesis in transgenic mice. Mol Endocrinol. 1990;4:597–603. doi: 10.1210/mend-4-4-597. [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woelfle JF, Harz K, Roth C. Modulation of circulating IGF-I and IGFBP-3 levels by hormonal regulators of energy homeostasis in obese children. Exp Clin Endocrinol Diabetes. 2007;115:17–23. doi: 10.1055/s-2006-957350. [DOI] [PubMed] [Google Scholar]
- Wolfe A, Kim HH, Stafford DEJ, Tobet SA, Radovick S. Identification of a discrete promoter region of the human GnRH gene that is sufficient for directing neuron specific expression: A role for POU homeodomain transcription factors. Mol Endo. 2002;16:435–449. doi: 10.1210/mend.16.3.0780. [DOI] [PubMed] [Google Scholar]
- Wolfe AM, Wray S, Westphal H, Radovick S. Cell-specific expression of the human gonadotropin-releasing hormone gene in transgenic animals. J Biol Chem. 1995;271:20018–20023. doi: 10.1074/jbc.271.33.20018. [DOI] [PubMed] [Google Scholar]
- Wray S, Grant P, Gainer H. Evidence that Cells Expressing Luteinizing Hormone-Releasing Hormone mRNA in the Mouse are Derived from Progenitor Cells in the Olfactory Placode. Proc Natl Acad Sci USA. 1989;86:8132–8136. doi: 10.1073/pnas.86.20.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S, Hoffman G. Postnatal morphological changes in rat LHRH neurons correlated with sexual maturation. Neuroendocrinology. 1986;43:93–97. doi: 10.1159/000124516. [DOI] [PubMed] [Google Scholar]
- Wu S, Divall SA, Nwaopara A, Radovick S, Wondisford FE, Ko C, Wolfe A. Obesity induced infertility and hyperandrogenism are corrected by deletion of the insulin receptor in the ovarian theca cell. Diabetes. 2013 doi: 10.2337/db13-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Divall S, Hoffman GE, Le WW, Wagner KU, Wolfe A. Jak2 is necessary for neuroendocrine control of female reproduction. J Neurosci. 2011;31:184–192. doi: 10.1523/JNEUROSCI.2974-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Divall S, Wondisford F, Wolfe A. Reproductive tissues maintain insulin sensitivity in diet-induced obesity. Diabetes. 2012;61:114–123. doi: 10.2337/db11-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia YX, Weiss JM, Polack S, Diedrich K, Ortmann O. Interactions of insulin-like growth factor-I, insulin and estradiol with GnRH-stimulated luteinizing hormone release from female rat gonadotrophs. Eur J Endocrinol. 2001;144:73–79. doi: 10.1530/eje.0.1440073. [DOI] [PubMed] [Google Scholar]
- Yang R, Wilcox DM, Haasch DL, Jung PM, Nguyen PT, Voorbach MJ, Doktor S, Brodjian S, Bush EN, Lin E, et al. Liver-specific knockdown of JNK1 up-regulates proliferator-activated receptor gamma coactivator 1 beta and increases plasma triglyceride despite reduced glucose and insulin levels in diet-induced obese mice. J Biol Chem. 2007;282:22765–22774. doi: 10.1074/jbc.M700790200. [DOI] [PubMed] [Google Scholar]
- Yoon H, Enquist LW, Dulac C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell. 2005;123:669–682. doi: 10.1016/j.cell.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Zanger K, Radovick S, Wondisford FE. CREB binding protein recruitment to the transcription complex requires growth factor-dependent phosphorylation of its GF box. Mol Cell. 2001;7:551–558. doi: 10.1016/s1097-2765(01)00202-7. [DOI] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Levine JE, Ronnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone neurons express K(ATP) channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci. 2007;27:10153–10164. doi: 10.1523/JNEUROSCI.1657-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen S, Zakaria M, Wolfe A, Radovick S. Regulation of gonadotropin-releasing hormone (GnRH) gene expression by insulin-like growth factor I in a cultured GnRH-expressing neuronal cell line. Mol Endocrinol. 1997;11:1145–1155. doi: 10.1210/mend.11.8.9956. [DOI] [PubMed] [Google Scholar]
- Zhou XY, Shibusawa N, Naik K, Porras D, Temple K, Ou H, Kaihara K, Roe MW, Brady MJ, Wondisford FE. Insulin regulation of hepatic gluconeogenesis through phosphorylation of CREB-binding protein. Nat Med. 2004;10:633–637. doi: 10.1038/nm1050. [DOI] [PubMed] [Google Scholar]