Abstract
Researchers have proposed that estrogen deficiency will lead to a Sjögren's syndrome (SjS)-like lacrimal gland inflammation, aqueous tear deficiency and dry eye. The purpose of this study was to determine whether this proposal is correct. Lacrimal glands were obtained from adult, age-matched wild type (WT) and aromatase knockout (ArKO) mice, in which estrogen synthesis is completely eliminated. Tissues were also obtained from autoimmune MRL/Mp-lpr/lpr (MRL/lpr) mice as inflammation controls. Tear volumes in WT and ArKO mice were measured and glands were processed for molecular biological and histological evaluation. Our results demonstrate that estrogen absence does not lead to a SjS-like inflammation in lacrimal tissue or to an aqueous-deficient dry eye. There was no upregulation of genes associated with inflammatory pathways in lacrimal glands of male or female ArKO mice. Such inflammatory activity was prominent in autoimmune MRL/lpr tissues. We also found no evidence of inflammation in lacrimal gland tissue sections of estrogen-deficient mice, and tear volumes of ArKO males were actually increased as compared to those WT controls. Interestingly, our study did show that estrogen absence influences the expression of thousands of lacrimal gland genes, and that this impact is sex- and genotype-specific. Our findings demonstrate that estrogen absence is not a risk factor for the development of SjS-like lacrimal gland inflammation or for aqueous-deficient dry eye in mice.
Keywords: lacrimal gland, mice, estrogen, male, female, sex difference, inflammation, tear volume, gene expression
1. Introduction
The lacrimal gland is the primary source of the tear film's aqueous layer. This gland secretes proteins, electrolytes and water that collectively play a prominent role in maintaining ocular surface integrity, protecting against microbial challenge and preserving visual acuity (Sullivan, 2004). Malfunction of the lacrimal gland, in turn, leads to aqueous tear deficiency. An example is Sjögren's syndrome (SjS), an autoimmune disease characterized by extensive glandular inflammation, immune-mediated dysfunction and/or destruction of acinar and ductal epithelial cells, a precipitous decline in aqueous tear output, and consequent dry eye disease (Sullivan, 2004).
It is important to note that dry eye disease occurs predominantly in women (2007). This prevalence has been linked to estrogen status. Investigators have proposed that estrogens positively modulate the structure, function and secretion of the lacrimal gland and that estrogen deficiency leads to numerous lacrimal gland sequelae, including acinar cell disruption, apoptosis and necrosis, cellular vacuolization, DNA degradation, inflammation, glandular regression and aqueous-deficient dry eye (Azzarolo et al., 2003; Konttinen et al., 2012; Mostafa et al., 2012; Song et al., 2012; Sullivan, 20041). Researchers have also posited that estrogen insufficiency promotes a SjS-like autoimmune exocrinopathy (Arakaki et al., 2010; Hayashi et al., 2004; Ishimaru et al., 2003; Ishimaru et al., 1999; Shim et al., 2004; Sullivan, 2004; Takahashi et al., 1997). Conversely, estrogen treatment purportedly corrects such defects in lacrimal gland anatomy and physiology, and promotes aqueous secretion (Affinito et al., 2003; Guaschino et al., 2003; Mostafa et al., 2012; Sullivan, 2004).
If these investigators' proposals are correct, we would predict that complete estrogen absence would induce an upregulation of inflammatory pathways in the lacrimal gland, a SjS-like inflammation in lacrimal tissue, and aqueous tear deficiency. To test this prediction, we examined molecular biological and histological attributes of the lacrimal glands, as well as tear volumes, of male and female aromatase knockout (ArKO) mice. These animals were generated by the targeted disruption of exon IX in the cyp19 gene and have no aromatase, the cytochrome P450 enzyme that controls the formation of estrogens. Consequently estrogen synthesis is completely eliminated in ArKO mice (Fisher et al., 1998).
2. Materials & Methods
2.1. Animals and tissue collections
Breeding pairs of C57BL/6J - aromatase knockout (ArKO) heterozygous mice were acquired from Dr. Orhan Oz (University of Texas Southwestern Medical Center, Dallas, TX). Animals were sent to Charles River Breeding Laboratories (Wimington, MA) for initial quarantine, health monitoring and serology, and then shipped to the Animal Facilities of the Schepens Eye Research Institute (Boston, MA). Mice were maintained and bred in constant temperature rooms with fixed light/dark intervals of 12 hours length. Offspring were genotyped by following a published protocol (Fisher et al., 1998). In brief, genomic DNA was isolated from tails by utilizing a GenEluteTM Mammalian Genomic DNA Miniprep Kit (Sigma-Aldrich, Saint Louis, MO). The DNA was amplified by PCR with a Hybaid OMN-E thermocycler (Thermo Electron Corp, Burlington, Ontario, Canada) by employing exon 9 gene primers (forward: GTGACAGAGACATAAAGATCG; reverse: GTAAATTCATTGGGCTTAGGG) and neo gene primers (forward: ATCAGGATGATCTGGACGAAGA; reverse: CCACAGTCGATGAATCCAGAA). The PCR conditions were 1 cycle (3 minutes at 94°C), 35 cycles (40 second at 94°C, 30 second at 55°C, 45 second at 72°C) and 1 cycle (5 minutes at 72°C) and amplicons were examined on 2.5% agarose gels. Band sizes equaled 220 bp from wild type (WT) mice, 170 bp from ArKO mice, and both fragments from heterozygotes. In addition to these animals, adult female and male MRL/Mp-lpr/lpr (MRL/lpr) mice were obtained from Jackson Laboratories (Bar Harbor, ME) to serve as controls.
When indicated, mice were sacrificed by CO2 inhalation and lacrimal glands were removed for either molecular biological procedures or histology. Glandular samples were prepared by combining tissues from 5 to 6 mice/sex/group. Three different sample preparations were made for each tissue/sex/group and then processed for RNA analysis.
For histological evaluation, tissues were fixed in 10% buffered formalin, dehydrated, embedded in methacrylate, cut into 3 μm sections and stained with hematoxylin and eosin or with Periodic-acid Schiff, according to previously described techniques (Sato and Sullivan, 1994). Sections were taken from different tissue areas, all separated by minimal distances of at least 30 μm. Slides were observed with an Olympus BH-2 light microscope and micrographs were obtained with a Nikon Eclipse E800 and SPOT camera (MicroVideo Instruments Inc., Avon, MA).
All research studies with mice were approved by the Institutional Animal Care and Use Committee of The Schepens Eye Research Institute and adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
2.2. Measurement of aqueous tear production
Tear volumes were measured by using a modified phenol red thread test (Zone-Quick™, Lacrimedics, Eastsound, WA) (Barabino et al., 2005). Under direct visualization with a biomicroscope, meniscal tears were removed and then threads were placed in the lateral canthus of the right eye for 30 seconds. The tear migration distance in mm was determined by using a microscope (Zeiss S4, West Germany) and a hemocytometer. Tears measurements were repeated 3 times and the results were averaged. Statistical analyses of these data were performed with Student's t-test (two-tailed, unpaired).
2.3. Molecular biological procedures
Total RNA was extracted from lacrimal glands by using TRIzol reagent (Invitrogen Corp., Carlsbad, CA) and purified with RNAqueous spin columns (Ambion, Austin, Tx). The glandular RNA samples were treated with RNase-free DNase (Invitrogen), analyzed spectrophotometrically at 260 nm to determine concentration and assessed with a RNA 6000 Nano LabChip and an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA) to verify RNA integrity. The RNA samples were then stored at -80°C until processing.
Gene expression was determined by the use of two procedures. One involved the processing of RNA samples for hybridization to CodeLink UniSet Mouse 20K I Bioarrays (n ∼ 20,000 genes/array; Amersham Biosciences/GE Healthcare, Piscataway, NJ), according to reported procedures (Richards et al., 2006). In brief, cDNA was synthesized from RNA (2 μg) with a CodeLink Expression Assay Reagent Kit (Amersham) and purified with a QIAquick purification kit (Qiagen, Valencia, CA). Samples were then dried, and cRNA was generated with a CodeLink Expression Assay Reagent Kit (Amersham), recovered with an RNeasy kit (Qiagen) and quantified with an UV spectrophotometer. Fragmented and biotin-labeled cRNA was incubated and shaken at 300 rpm on a CodeLink Bioarray at 37°C for 18 hours. After this time period, the Bioarray was washed, exposed to streptavidin-Alexa 647, and scanned by utilizing ScanArray Express software and a ScanArray Express HT scanner (Packard BioScience, Meriden, CT) with the laser set at 635 nm, laser power at 100%, and photomultiplier tube voltage at 60%. Scanned image files were examined by using CodeLink image and data analysis software (Amersham), which yielded both raw and normalized hybridization signal intensities for each array spot. The intensities of the ∼ 20,000 spots on the Bioarray image were normalized to a median of 1. Standardized data, with signal intensities exceeding 0.50, were analyzed with sophisticated bioinformatic software (Geospiza, Seattle, WA). This comprehensive software also generated gene ontology, KEGG (i.e. Kyoto Encyclopedia of Genes and Genomes) pathway and z-score reports. The ontologies were organized according to the guidelines of the Gene Ontology Consortium (http://www.geneontology.org/GO.doc.html) (Ashburner et al., 2000).
The other procedure to analyze gene expression involved the hybridization of each cRNA (20 μg) sample to a GeneChip Mouse Genome 430A 2.0 Array (Affymetrix, Santa Clara, CA) according to the manufacturer's protocol. Hybridized GeneChips were scanned with an Affymetrix Model 700 Scanner and expression data files were generated from array images using Affymetrix Microarray Suite 4.0 software. GeneChip data were standardized by selecting the default scaling in Affymetrix GeneChip Operating Software, which yields a trimmed mean intensity of 500 for each GeneChip microarray. Normalized data with a quality value of 1.0 were then evaluated with Geospiza Genesifter software.
Gene expression data were assessed without log transformation and statistical analyses of these data were performed with Student's t-test (two-tailed, unpaired) by using the GeneSifter software. Our statistical approaches were not tailored for multiple comparisons. Genes that were up- or down-regulated similarly in different groups were identified by using “Unigene” accession numbers and an intersector program (Geospiza; www.public.genesifter.net). Data from the individual CodeLink and Affymetrix arrays are accessible for free download through the National Center for Biotechnology Information's Gene Expression Omnibus (http://www.genesifter.net/web/dataCenter.html) via series accession numbers GSE5878 and GSE5876.
For results presented in this paper, we focused on data from Affymetrix GeneChips, and used the CodeLink Bioarray information for confirmation of selected gene expression. We addressed the comparative merit of these microarrays in previous publications (e.g. Rahimi Darabad et al., 2013).
3. Results
3.1. Influence of complete estrogen absence on gene expression in male and female lacrimal glands
To determine whether complete estrogen absence induces an upregulation of inflammatory pathways in the lacrimal gland, tissues were obtained from adult, age-matched male and female WT (male = 22.7 ± 0.9 weeks; female = 21.4 ± 1.0 weeks) and ArKO (male = 21.6 ± 1.0 weeks; female = 21.7 ± 1.1 weeks) mice (n = 5/sex/group; n = 3 separate experiments). For control purposes, lacrimal glands were also collected from adult, autoimmune female and male MRL/lpr mice (n = 6/group; n = 3 experiments). Glands were pooled according to sex and group, processed for the isolation of total RNA, and evaluated for differentially expressed mRNAs by using Affymetrix GeneChips and Geospiza bioinformatics software.
Our findings demonstrate that estrogen absence does not induce an upregulation of inflammatory pathways in the lacrimal gland. There were 73 and 75 significantly upregulated biological process ontologies (n = ≥ 12 genes/ontology) in the male and female ArKO lacrimal glands, respectively (e.g. Table 1). None of these ontologies were immune-related. Similarly, there were 12 and 16 significantly upregulated KEGG pathways in the male and female ArKO tissues (e.g. Table 2). Of these, only one pathway, Fcγ receptor-mediated phagocytosis (n = 8 genes) was significantly increased in the male. In contrast, the autoimmune female MRL/lpr lacrimal glands contained 364 significantly upregulated biological process ontologies (n ≥ 12 genes/group), of which 81 were immune-related. Examples included those associated with the immune system process (n = 147 ↑genes; z-score [zsc] = 8.24), defense response (85 ↑genes; zsc = 6.37), leukocyte activation (67 ↑genes; zsc = 6.93), lymphocyte activation (57 ↑genes; zsc = 6.32), T cell activation (42 ↑genes; zsc = 6.03), inflammatory response (44 ↑genes; zsc = 5.12), innate immune response (40 ↑genes; zsc = 5.15) and response to cytokine stimulus (36 ↑genes; zsc = 5.15). In addition, 9 out of 22 significantly upregulated KEGG pathways in the MRL/lpr lacrimal glands had an immune basis (e.g. chemokine signaling pathway, 26 ↑genes; zsc = 2.43). Clearly, the lacrimal glands of estrogen-deficient mice were not immunologically analogous to those of the autoimmune animals.
Table 1. Upregulation of biological process ontologies in the lacrimal glands of male and female mice with complete estrogen absence.
| Ontology | Genes↑ | z-score |
|---|---|---|
| Female | ||
| Monocarboxylic acid catabolic process | 10 | 4.96 |
| Oxidation-reduction process | 56 | 4.6 |
| Fatty acid catabolic process | 9 | 4.54 |
| Coenzyme metabolic process | 20 | 4.42 |
| Cellular respiration | 10 | 4.37 |
| Cofactor metabolic process | 23 | 4.31 |
| Carboxylic acid catabolic process | 15 | 4.23 |
| Organic acid catabolic process | 15 | 4.23 |
| Male | ||
| Glutathione metabolic process | 7 | 5.65 |
| Sulfur compound metabolic process | 12 | 4.16 |
| Lipid biosynthetic process | 25 | 3.99 |
| Small molecule biosynthetic process | 34 | 3.91 |
| Peptide metabolic process | 7 | 3.91 |
| Small molecule metabolic process | 90 | 3.66 |
| Regulation of protein complex assembly | 11 | 3.55 |
| Regulation of protein polymerization | 8 | 3.43 |
Biological process ontologies (≥ 7 genes/sex) with the highest z-scores in lacrimal glands of ArKO mice are listed. A z-score is a statistical rating of the relative expression of genes, and demonstrates how much they are over- or under-represented in a given gene list (Doniger et al., 2003). Positive z scores reflect a higher number of genes meeting the criterion than is expected by chance, whereas negative z scores represent fewer genes meeting the criterion than expected by chance (Doniger et al., 2003). Z-scores with values > 2.0 are significant. Terms: Genes↑ -number of genes up-regulated in ArKO glands; z-score - specific score for the up-regulated genes in the ArKO tissues.
Table 2. Upregulation of KEGG pathways in the lacrimal glands of male and female mice with complete estrogen absence.
| KEGG pathway | Genes↑ | z-score |
|---|---|---|
| Female | ||
| Metabolic pathways | 88 | 4.75 |
| Valine, leucine and isoleucine degradation | 10 | 4.57 |
| Alzheimer's disease | 20 | 3.81 |
| Peroxisome | 11 | 3.44 |
| Fatty acid metabolism | 7 | 3.11 |
| Glycerolipid metabolism | 7 | 2.96 |
| Oxidative phosphorylation | 13 | 2.72 |
| Male | ||
| Amino sugar and nucleotide sugar | ||
| metabolism | 6 | 3.75 |
| Metabolic pathways | 57 | 3.49 |
| Glycolysis / Gluconeogenesis | 7 | 3.29 |
| Fcγ receptor-mediated phagocytosis | 8 | 2.75 |
| Fructose and mannose metabolism | 4 | 2.64 |
| Pyrimidine metabolism | 8 | 2.49 |
| Glutathione metabolism | 5 | 2.2 |
KEGG pathways (≥ 7 genes/sex) with the highest z-scores in lacrimal glands of ArKO mice are listed.
This lack of inflammatory activity in lacrimal tissues of mice with estrogen deficiency does not reflect a simple lack of response to this change in hormonal environment. Indeed, the absence of estrogen exerted a significant impact on the expression of thousands of genes (Tables 3 and 4). Of particular interest, this influence of estrogen deficiency on lacrimal gland gene expression was sex-dependent. As shown in Table 5, less than 8.7% of the genes significantly affected by estrogen absence were the same in males and females. Further, less than 20.3% of the biological process ontologies (≥ 12 genes/ontology) were similar in both sexes (data not shown). In effect, the vast majority of gene transcripts that were significantly altered in complete estrogen absence were sex-specific.
Table 3. Effect of complete estrogen absence on genotype- and sex-related gene expression in female and male mouse lacrimal glands.
| Genes | |||
|---|---|---|---|
| Group | WT > ArKO | ArKO > WT | Total |
|
| |||
| Female | 978 | 770 | 1,748 |
| Male | 525 | 542 | 1,067 |
| Male + Female | 516 | 335 | 851 |
|
| |||
| Group | M>F | F>M | Total |
|
| |||
| WT | 1,176 | 1,199 | 2,375 |
| ArKO | 1,258 | 1,278 | 2,536 |
| WT + ArKO | 1,590 | 1,585 | 3,175 |
The expression of listed genes was significantly (p < 0.05) upregulated between the groups. Abbreviations: WT = wild type; M = male; F = female
Table 4. Impact of complete estrogen absence on gene expression ratios in the mouse lacrimal gland.
| Gene Identity | Gene | Ratio | p value | Ontology |
|---|---|---|---|---|
| F, WT > ArKO | ||||
| Reep6 | Receptor accessory protein 6 | 17.3 | 0.0002 | integral to membrane |
| Tulp2 | Tubby like protein 2 | 8.0 | 0.0047 | hydrolase activity |
| Abcb1a | ATP-binding cassette, subfamily B, member 1A | 7.9 | 0.0069 | ATP catabolic process |
| Gdf5 | Growth differentiation factor 5 | 6.5 | 0.0186 | growth |
| Slc39A8 | Solute carrier family 39, member 8 | 6.3 | 0.0005 | transport |
| Snap91 | Synaptosomal-associated protein 91 | 5.2 | 0.0020 | synaptic transmission |
| F, ArKO>WT | ||||
| Ly6f | Lymphocyte antigen 6 complex, locus F | 75.8 | 0.0031 | anchored to membrane |
| Cyp2j13 | Cytochrome P450, family 2, subfamily j, polypeptide 13 | 74.8 | 0.0137 | monooxygenase activity |
| Mrap | Melanocortin 2 receptor accessory protein | 61.3 | 0.0000 | positive regulation of cAMP biosynthetic process |
| Cuzd1 | CUB and zona pellucida-like domains 1 | 31.2 | 0.0051 | cell cycle |
| AK016936 | ribosomal modification protein rimK-like family member B | 22.3 | 0.0074 | protein modification process |
| Slc12a1 | Solute carrier family 12, member 1 | 15.7 | 0.0159 | transport |
| M, WT > ArKO | ||||
| B4galt1 | UDP-Gal:betaGlcNAc beta1,4- galactosyltransferase, polypeptide 1 | 9.4 | 0.0002 | epithelial cell development |
| Fgl2 | Fibrinogen-like protein 2 | 3.4 | 0.0017 | signal transduction |
| Cuzd1 | CUB and zona pellucida-like domains 1 | 3.3 | 0.0493 | cell cycle |
| Prlr | Prolactin receptor, mRNA | 3.1 | 0.0100 | JAK-STAT cascade |
| Sertad1 | SERTA domain containing 1 | 3.1 | 0.0310 | transcription, DNA-dependent |
| Cnn3 | Calponin 3, acidic | 3.0 | 0.0387 | actomyosin structure organization |
| M, ArKO > WT | ||||
| Nmu | Neuromedin U | 471.5 | 0.0110 | gastric acid secretion |
| Ddc | Dopa decarboxylase | 302.1 | 0.0024 | cellular amino acid metabolic process |
| Smr2 | Submaxillary gland androgen regulated protein 2 | 51.1 | 0.0371 | response to toxin |
| Klk1b24 | Kallikrein 1-related peptidase b24 | 27.9 | 0.0233 | proteolysis |
| Bmp6 | Bone morphogenetic protein 6 | 20.3 | 0.0045 | eye development |
| Pcdh20 | Protocadherin 20 | 17.9 | 0.0312 | cell adhesion |
Relative ratios were calculated from Affymetrix data by comparing the degree of gene expression in lacrimal glands from male (M), female (F), WT and ArKO groups. Genes listed had a signal intensity of > 100 in at least one group, a comparative p value (between treatments) of < 0.05 and a known identity. Gene identities are shown as gene symbols, except for ribosomal modification protein rimK-like family member B. This gene does not yet have symbol, and the gene accession number is listed.
Table 5. Effect of estrogen absence and sex on gene expression in the mouse lacrimal gland.
| Comparison 1 | Comparison 2 | Total Genes | Common Genes | Common/Total (%) |
|---|---|---|---|---|
| F, WT>ArKO | M, WT>ArKO | 1,211 | 86 | 7.1 |
| F, ArKO>WT | M, ArKO>WT | 938 | 81 | 8.6 |
| WT, M>F | ArKO, M>F | 617 | 587 | 23.6 |
| WT, F > M | ArKO, F>M | 706 | 670 | 25.5 |
Common genes were identified by using the Geospiza Intersector program. The expression of genes in the “Total” column was significantly (p < 0.05) upregulated in the designated comparison group.
The absence of estrogens also influenced the nature of sex-associated differences in lacrimal gland gene expression. This effect was genotype-specific. As demonstrated in Table 5, over 74% of genes upregulated in male or female tissues were unique to either WT or ArKO mice. However, despite these specific gene differences, numerous upregulated ontologies and pathways were similar in female WT and ArKO mice. Thus, 35% of the 40 highest, significant z-scores of biological process ontologies (n = 12 genes/ontology) in female ArKO lacrimal tissues were significantly increased in the WT females. In addition, 52.4% of all upregulated KEGG pathways in female ArKO lacrimal glands were also elevated in WT female tissues. These similarities were not found in male WT and ArKO mice.
To confirm in part the Affymetrix GeneChip results, gene expression was analyzed by the use of CodeLink Bioarrays. This experimental approach verified the effects of complete estrogen absence on a number of genes (Table 6). The CodeLink Biorray data also showed no evidence of any upregulation of inflammatory ontologies (n = ≥ 3 genes/ontology) in lacrimal tissues of male or female ArKO mice.
Table 6. Verification of selected GeneChip gene expression results.
| Ratio | |||||
|---|---|---|---|---|---|
|
| |||||
| Gene Identity | Gene | WT, Affy | ArKO, Affy | WT, CL | ArKO, CL |
| Female > Male | |||||
| Asgr1 | Asialoglycoprotein receptor 1 | 56.8 | 88.5 | 46.0 | 60.9 |
| Cyp2a5 | Cytochrome P450, family 2, subfamily a, polypeptide 4 | 9.2 | 15.2 | 14.0 | 10.1 |
| Prlr | Prolactin receptor | 4.4 | 10.6 | 2.9 | 5.3 |
| Lap3 | Leucine aminopeptidase 3 | 3.1 | 3.8 | 2.9 | 3.6 |
| Ccni | Cyclin I | 2.9 | 1.5 | 2.7 | 2.4 |
| Gstm1 | Glutathione S-transferase, μ 1 | 2.1 | 1.9 | 2.0 | 2.8 |
| Male > Female | |||||
| Eif2s3y | Eukaryotic translationinitiation factor 2,subunit 3 | 385.7 | 183.2 | 23.6 | 17.3 |
| Cyp2j13 | Cytochrome P450, family 2, subfamily j, polypeptide 13 | 156.9 | 2.5 | 553.9 | 2.9 |
| Pgf | Placental growth factor | 22.1 | 2.3 | 10.5 | 2.8 |
| Slc27a22 | Solute carrier family 27 (fatty acid transporter), member 2 | 9.1 | 1.6 | 14.2 | 3.0 |
| Ccl8 | Chemokine (C-C motif) ligand 8 | 5.2 | 5.7 | 7.1 | 5.5 |
| Anxa5 | Annexin A5 | 2.1 | 1.5 | 1.8 | 1.8 |
The expressions of selected genes, that were significantly altered in lacrimal glands of WT and ArKO mice according to Affymetrix GeneChip analyses, were reevaluated with CodeLink Bioarrays. Numbers represent the relative increase in gene expression. Abbreviations: Imm – Immortalized; Affy – Affymetrix; CL – CodeLink
The lacrimal gland genes significantly influenced by complete estrogen absence were very different than those recently discovered in meibomian glands of ArKO mice (Darabad et al., 2013). Comparison of the meibomian gland dataset (NCBI GEO #GSE5878) to that of the present study showed that at least 92% of the meibomian and lacrimal gland genes were different (Table 7).
Table 7. Comparative gene expression between lacrimal and meibomian glands of WT and ArKO mice.
| Comparison | MeibG genes different | LG genes different | Common genes | Common genes (%) |
|---|---|---|---|---|
| F, WT>ArKO | 745 | 622 | 80 | 5.5 |
| F, ArKO>WT | 779 | 593 | 45 | 3.2 |
| M, WT>ArKO | 706 | 420 | 52 | 4.4 |
| M, ArKO>WT | 438 | 328 | 24 | 3.0 |
| WT, M>F | 1,398 | 817 | 158 | 6.7 |
| WT, F>M | 923 | 636 | 98 | 5.9 |
| ArKO, M>F | 912 | 852 | 153 | 8.0 |
| ArKO, F>M | 950 | 698 | 107 | 6.1 |
Lacrimal gland (“LG”) Affymetrix data were from the present study, whereas meibomian gland (“MeibG”) Affymetrix data were obtained from a recently published dataset (Rahimi Darabad et al., 2013). Comparisons were made with the Geospiza Intersector program. The percentage of common genes was determined by dividing the number of common genes by the total number of lacrimal and meibomian gland genes and multiplying by 100.
3.2. Effect of complete estrogen absence on the histology of male and female lacrimal glands
To examine whether complete estrogen induces an SjS-like inflammation in lacrimal tissue, glands (n = 4/sex/strain) from adult, age-matched male and female WT (male = 18.1 ± 0 weeks; female = 17.9 ± 0.3 weeks) and ArKO (male = 17.4 ± 0.7 weeks; female = 17.6 ± 0.3 weeks) mice were processed for histological analysis. As shown in Figure 1, we found no evidence of inflammation in the lacrimal glands of estrogen-deficient male or female mice. In addition, complete estrogen absence had no obvious influence on the histological appearance of any of the tissue sections.
Figure 1.
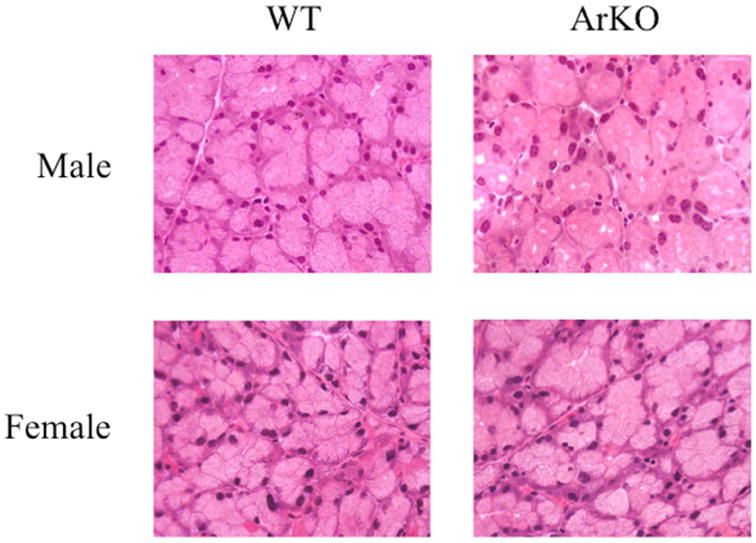
Representative light micrographs of male and female WT and ArKO lacrimal glands. There were no obvious histological differences between WT and ArKO glands. Sections were stained with hematoxylin and eosin. All panels are 40 × magnification.
3.3. Impact of complete estrogen absence on the tear volumes of male and female mice
To determine whether complete estrogen absence causes an aqueous tear deficiency, tear volumes were measured in WT and ArKO mice (n = 5/sex/strain; 20.2 ± 1.9 weeks old). Our results demonstrated that estrogen deficiency caused a significant (p < 0.005) increase in the tear volume of male mice (WT = 1.00 ± 0.03; ArKO = 1.58 ± 0.05). In contrast, this hormonal change had no effect on the tear volume of female mice (WT = 1.13 ± 0.05; ArKO = 1.19 ± 0.10). The tear volume of male ArKO mice was also greater than that of female ArKO mice (p < 0.01).
4. Discussion
Our study demonstrates that complete estrogen absence does not induce an upregulation of inflammatory pathways in the lacrimal gland, does not elicit a SjS-like inflammation in lacrimal tissue, and does not cause an aqueous tear deficiency in mice. Our research does show that estrogen absence influences the expression of thousands of lacrimal gland genes, and that this impact is sex- and genotype-specific. Overall, our data demonstrate that the lacrimal glands of estrogen-deficient male and female mice are functional and appear histologically normal.
Our findings are not in agreement with those linking estrogen deficiency with lacrimal gland pathophysiology and dry eye disease (Arakaki et al., 2010; Azzarolo et al., 2003; Gagliano et al., 2014; Hayashi et al., 2004; Ishimaru et al., 2003; Ishimaru et al., 1999; Mostafa et al., 2012; Shim et al., 2004; Sullivan, 2004; Takahashi et al., 1997). This lack of agreement is not surprising. The published literature concerning estrogen influence on the lacrimal gland is replete with conflicting results. For example, a number of investigators have reported that neither estrogen insufficiency nor estrogen treatment has any impact on the size, morphology, total protein content, specific enzyme activity, lymphocyte accumulation or secretion of the lacrimal gland (Kuscu et al., 2003; Pelit et al., 2003; Sullivan, 2004). And yet other researchers have found that estrogens have a negative effect on lacrimal tissue and promote glandular regression, suppression of protein production, androgen antagonism and reduced tear output (Ishimaru et al., 1999; Sato and Sullivan, 1994; Sullivan, 2004). Some of these disparate findings regarding estrogens might be explained by differences in experimental design, hormone dosage or animal model (Sullivan, 2004; Truong et al, 2014). It is also possible that these conflicting results may be due to variations in the relative levels of progesterone, a steroid that may significantly modify estrogen effects. However, despite this lack of consensus, our data clearly demonstrate that estrogen absence does not cause an autoimmune exocrinopathy in the lacrimal gland, or an aqueous-deficient dry eye, in mice.
Our findings are also not in agreement with those of Shim et al. (2004), who proposed that ArKO mice develop an SjS phenotype. These investigators reported an extensive inflammation in salivary glands of both male and female ArKO mice between the ages of 12 to 17 months. Given that our mice were approximately 5 months of age, it may be that our different results are due to animal age. It is also possible, though, as found in SjS mouse models that the magnitude of inflammation in salivary and lacrimal glands may differ significantly. For example, male non-obese diabetic (NOD) mice have extensive inflammation in their lacrimal tissues, but essentially none in their salivary glands. Female NOD mice feature tremendous inflammation in their salivary, but not lacrimal, glands. MRL/lpr female mice have inflamed lacrimal and salivary glands, whereas males have far less, if any, immunopathology. In effect, inflammatory changes in salivary tissue are not necessarily mirrored in lacrimal glands (Toda et al., 1999).
Are estrogens, then, involved at all in the lacrimal tissue dysfunction in SjS? At present, the precise role of estrogens in the etiology of lacrimal gland inflammation and aqueous-deficient dry eye in SjS is unknown. Estrogens have been shown to accelerate (Homo-Delarche et al., 1991; Olsen and Kovacs, 1996; Roubinian et al., 1978; Talal, 1978), and anti-estrogens (Auborn et al., 2003; Nelson and Steinberg, 1987; Sthoeger et al., 2003; Wu et al., 2000) and selective estrogen receptor modulators (Apelgren et al., 1996) to retard, systemic disease processes in mouse models of SjS. Estrogens are also known to enhance the polyclonal B cell activation, autoantibody formation and tissue abnormalities encountered in SjS (Ahmed et al., 1989; Carlsten et al., 1990; Sato and Sullivan, 1994). As further considerations, estrogens often promote inflammation (Choubey and Moudgil, 2011; Whitacre, 2001), upregulate pro-inflammatory gene expression in lacrimal tissue (Suzuki et al., 2006), stimulate the accumulation of lymphoid infiltrates in MRL/lpr lacrimal glands (Sato and Sullivan, 1994), and increase the prevalence of dry eye disease in women (Schaumberg et al., 2001). Given this background, it could be that estrogens have a deleterious impact on lacrimal gland function and reduce aqueous secretion. Consistent with this possibility are some (Sato and Sullivan, 1994; Sullivan, 2004; Toda et al., 1999), but not other (Affinito et al., 2003; Arakaki et al., 2010; Azzarolo et al., 2003; Forsblad-d'Elia et al., 2009; Guaschino et al., 2003; Hayashi et al., 2004; Ishimaru et al., 2003; Ishimaru et al., 1999; Kuscu et al., 2003; Mostafa et al., 2012; Pelit et al., 2003; Shim et al., 2004; Sullivan, 2004; Takahashi et al., 1997; Taner et al., 2004), research findings. Indeed, with the possible exception of specific genes (Suzuki et al., 2006), no consensus exists concerning the cellular targets for, or the cellular processes that may be regulated by, estrogens in lacrimal tissue (Affinito et al., 2003; Azzarolo et al., 2003; Imbert et al., 2009; Ishimaru et al., 2008; Kuscu et al., 2003; Paliwal and De, 2006; Pelit et al., 2003; Sato and Sullivan, 1994; Seamon et al., 2008; Srikantan and De, 2008; Srikantan et al., 2007; Sullivan, 2004; Suzuki et al., 2006; Taner et al., 2004; Zylberberg et al., 2007). In fact, it has not yet even been established whether estrogens have functional receptors in the lacrimal gland.
We discovered that complete estrogen absence is associated with a significant increase in the tear volume of male, but not female, mice. This male-specific effect may represent a sexually dimorphic response to the known elevations in the serum concentrations of testosterone, androstenedione, follicle-stimulating hormone, luteinizing hormone, prolactin or leptin in ArKO mice (Britt et al., 2001; Fisher et al., 1998; Jones et al., 2000; McPherson et al., 2001), or to hormone-independent processes (Isensee and Ruiz Noppinger, 2007; Rinn and Snyder, 2005), or to the influences of Y-linked genes or X inactivation in the ArKO strain (Disteche et al., 2002; Migeon, 2006; Ostrer, 2001; Xu and Disteche, 2006).
We found that complete estrogen absence impacts the expression of thousands of lacrimal gland genes, and that this effect was sex- and genotype-specific. The fact that estrogen deficiency influences the male lacrimal gland is consistent with research demonstrating that estrogen action is very important in other male tissues (Grumbach and Auchus, 1999; MacGillivray et al., 1998). Further, the sex- and genotype-specificity of the lacrimal gland response to estrogen absence is analogous to that of the meibomian gland (Darabad et al., 2013), although the affected genes are different. For comparison, other studies have shown that the nature of estrogen and/or aromatase control of the brain, liver, adipose tissue and serum leptin levels is also different in males and females (Chavez et al., 2009; Hewitt et al., 2003; Hewitt et al., 2004; Hill et al., 2004; Jones et al., 2000; Simpson et al., 2005). Moreover, we have previously demonstrated that the specific genes regulated by sex steroids in lacrimal and meibomian glands are typically not the same (Sullivan et al., 2009).
We also discovered that, although the lacrimal gland gene response to estrogen absence was genotype-specific, many of the upregulated ontologies and KEGG pathways were similar in female WT and ArKO mice. This finding would suggest that estrogens may not play a predominant role in the sex-related differences of the mouse lacrimal gland. This suggestion is consistent with our previous observation that estrogen with or without progesterone seems to contribute very little to the known sex-associated variations in gene expression of the lacrimal gland (Richards et al., 2006; Suzuki et al., 2006).
Lastly, it is important to restate that our research was performed in mice. Though there are many similarities between the endocrine and immune systems of mice and humans, there are also many differences in both health and disease. Consequently, further research is required to determine whether the inability of estrogen deficiency to induce lacrimal gland inflammation in mice is the same response as in humans (Luu-The and Labrie, 2010; Seok et al., 2013).
5. Conclusions
Our research shows that complete estrogen absence does not induce an upregulation of inflammatory pathways in the lacrimal gland, does not elicit a SjS-like inflammation in lacrimal tissue, and does not cause an aqueous tear deficiency in mice. Our study also demonstrates that complete estrogen absence influences the expression of thousands of lacrimal gland genes, and that this impact is sex- and genotype-specific. Overall, our results show that the lacrimal glands of estrogen-deficient male and female mice are functional and appear histologically normal.
Highlights.
investigators proposed that estrogen deficiency causes lacrimal gland inflammation
investigators proposed that estrogen deficiency causes aqueous-deficient dry eye
our research shows that estrogen absence does not cause lacrimal gland inflammation
our study shows that estrogen absence does not cause aqueous-deficient dry eye
lacrimal glands of estrogen-deficient mice are functional and appear normal
Acknowledgments
The authors would like to express their appreciation to Drs. Orhan Oz (Dallas, TX), Evan Simpson (Clayton, Victoria, Australia), Roderick V. Jensen (Blacksburg, VA), Britt Bromberg (New Orleans, LA), Ms. Alexia Thomas (Dallas, TX), Ms. Wendy R. Kam, Mr. Michael J. Lombardi, Ms. Patricia Rowley and Ms. Marie Ortega (Boston, MA) and Mr. Adam M. Papallo (Cambridge, MA) for their assistance. This research was supported by NIH grant R01EY05612 and the Margaret S. Sinon Scholar in Ocular Surface Research Fund
Footnotes
The “Sullivan, 2004” review contains over 40 additional references about estrogen's possible influence on the lacrimal gland. These papers were published between 1930 to 2002.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:93–107. doi: 10.1016/s1542-0124(12)70082-4. [DOI] [PubMed] [Google Scholar]
- 2.Affinito P, Di Spiezio Sardo A, Di Carlo C, Sammartino A, Tommaselli GA, Bifulco G, Loffredo A, Loffredo M, Nappi C. Effects of hormone replacement therapy on ocular function in postmenopause. Menopause. 2003;10:482–487. doi: 10.1097/01.GME.0000063568.84134.35. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed SA, Aufdemorte TB, Chen JR, Montoya AI, Olive D, Talal N. Estrogen induces the development of autoantibodies and promotes salivary gland lymphoid infiltrates in normal mice. J Autoimmun. 1989;2:543–552. doi: 10.1016/0896-8411(89)90187-x. [DOI] [PubMed] [Google Scholar]
- 4.Apelgren LD, Bailey DL, Fouts RL, Short L, Bryan N, Evans GF, Sandusky GE, Zuckerman SH, Glasebrook A, Bumol TF. The effect of a selective estrogen receptor modulator on the progression of spontaneous autoimmune disease in MRL lpr/lpr mice. Cell Immunol. 1996;173:55–63. doi: 10.1006/cimm.1996.0251. [DOI] [PubMed] [Google Scholar]
- 5.Arakaki R, Ishimaru N, Hayashi Y. Immunotherapeutic targets in estrogen deficiency-dependent Sjogren's syndrome-related manifestations. Immunotherapy. 2010;2:339–346. doi: 10.2217/imt.10.18. [DOI] [PubMed] [Google Scholar]
- 6.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auborn KJ, Qi M, Yan XJ, Teichberg S, Chen D, Madaio MP, Chiorazzi N. Lifespan is prolonged in autoimmune-prone (NZB/NZW) F1 mice fed a diet supplemented with indole-3-carbinol. J Nutr. 2003;133:3610–3613. doi: 10.1093/jn/133.11.3610. [DOI] [PubMed] [Google Scholar]
- 8.Azzarolo AM, Eihausen H, Schechter J. Estrogen prevention of lacrimal gland cell death and lymphocytic infiltration. Exp Eye Res. 2003;77:347–354. doi: 10.1016/s0014-4835(03)00120-9. [DOI] [PubMed] [Google Scholar]
- 9.Barabino S, Shen L, Chen L, Rashid S, Rolando M, Dana MR. The controlled-environment chamber: a new mouse model of dry eye. Invest Ophthalmol Vis Sci. 2005;46:2766–2771. doi: 10.1167/iovs.04-1326. [DOI] [PubMed] [Google Scholar]
- 10.Britt KL, Drummond AE, Dyson M, Wreford NG, Jones ME, Simpson ER, Findlay JK. The ovarian phenotype of the aromatase knockout (ArKO) mouse. J Steroid Biochem Mol Biol. 2001;79:181–185. doi: 10.1016/s0960-0760(01)00158-3. [DOI] [PubMed] [Google Scholar]
- 11.Carlsten H, Tarkowski A, Holmdahl R, Nilsson LA. Oestrogen is a potent disease accelerator in SLE-prone MRL lpr/lpr mice. Clin Exp Immunol. 1990;80:467–473. doi: 10.1111/j.1365-2249.1990.tb03311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chavez C, Gogos A, Jones ME, van den Buuse M. Psychotropic drug-induced locomotor hyperactivity and prepulse inhibition regulation in male and female aromatase knockout (ArKO) mice: role of dopamine D1 and D2 receptors and dopamine transporters. Psychopharmacology (Berl) 2009;206:267–279. doi: 10.1007/s00213-009-1604-6. [DOI] [PubMed] [Google Scholar]
- 13.Choubey D, Moudgil KD. Interferons in autoimmune and inflammatory diseases: regulation and roles. J Interferon Cytokine Res. 2011;31:857–865. doi: 10.1089/jir.2011.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Disteche CM, Filippova GN, Tsuchiya KD. Escape from X inactivation. Cytogenet Genome Res. 2002;99:36–43. doi: 10.1159/000071572. [DOI] [PubMed] [Google Scholar]
- 15.Doniger SW, Salomonis N, Dahlquist KD, Vranizan K, Lawlor SC, Conklin BR. MAPPFinder: using Gene Ontology and GenMAPP to create a global gene-expression profile from microarray data. Genome Biol. 2003;4:R7. doi: 10.1186/gb-2003-4-1-r7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci U S A. 1998;95:6965–6970. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forsblad-d'Elia H, Carlsten H, Labrie F, Konttinen YT, Ohlsson C. Low serum levels of sex steroids are associated with disease characteristics in primary Sjogren's syndrome; supplementation with dehydroepiandrosterone restores the concentrations. J Clin Endocrinol Metab. 2009;94:2044–2051. doi: 10.1210/jc.2009-0106. [DOI] [PubMed] [Google Scholar]
- 18.Gagliano C, Caruso S, Napolitano G, Malaguarnera G, Cicinelli MV, Amato R, Reibaldi M, Incarbone G, Bucolo C, Drago F, Avitabile T. Low levels of 17-β-oestradiol, oestrone and testosterone correlate with severe evaporative dysfunctional tear syndrome in postmenopausal women: a case-control study. Br J Ophthalmol. 2014;98:371–376. doi: 10.1136/bjophthalmol-2012-302705. [DOI] [PubMed] [Google Scholar]
- 19.Grumbach MM, Auchus RJ. Estrogen: consequences and implications of human mutations in synthesis and action. J Clin Endocrinol Metab. 1999;84:4677–4694. doi: 10.1210/jcem.84.12.6290. [DOI] [PubMed] [Google Scholar]
- 20.Guaschino S, Grimaldi E, Sartore A, Mugittu R, Mangino F, Bortoli P, Pensiero S, Vinciguerra A, Perissutti P. Visual function in menopause: the role of hormone replacement therapy. Menopause. 2003;10:53–57. doi: 10.1097/00042192-200310010-00009. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi Y, Arakaki R, Ishimaru N. Apoptosis and estrogen deficiency in primary Sjogren syndrome. Curr Opin Rheumatol. 2004;16:522–526. doi: 10.1097/01.bor.0000135450.78047.78. [DOI] [PubMed] [Google Scholar]
- 22.Hewitt KN, Boon WC, Murata Y, Jones ME, Simpson ER. The aromatase knockout mouse presents with a sexually dimorphic disruption to cholesterol homeostasis. Endocrinology. 2003;144:3895–3903. doi: 10.1210/en.2003-0244. [DOI] [PubMed] [Google Scholar]
- 23.Hewitt KN, Pratis K, Jones ME, Simpson ER. Estrogen replacement reverses the hepatic steatosis phenotype in the male aromatase knockout mouse. Endocrinology. 2004;145:1842–1848. doi: 10.1210/en.2003-1369. [DOI] [PubMed] [Google Scholar]
- 24.Hill RA, Pompolo S, Jones ME, Simpson ER, Boon WC. Estrogen deficiency leads to apoptosis in dopaminergic neurons in the medial preoptic area and arcuate nucleus of male mice. Mol Cell Neurosci. 2004;27:466–476. doi: 10.1016/j.mcn.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Homo-Delarche F, Fitzpatrick F, Christeff N, Nunez EA, Bach JF, Dardenne M. Sex steroids, glucocorticoids, stress and autoimmunity. J Steroid Biochem Mol Biol. 1991;40:619–637. doi: 10.1016/0960-0760(91)90285-d. [DOI] [PubMed] [Google Scholar]
- 26.Imbert Y, Foulks GN, Brennan MD, Jumblatt MM, John G, Shah HA, Newton C, Pouranfar F, Young WW., Jr MUC1 and estrogen receptor alpha gene polymorphisms in dry eye patients. Exp Eye Res. 2009;88:334–338. doi: 10.1016/j.exer.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Isensee J, Ruiz Noppinger P. Sexually dimorphic gene expression in mammalian somatic tissue. Gend Med. 2007;4(Suppl B):S75–95. doi: 10.1016/s1550-8579(07)80049-0. [DOI] [PubMed] [Google Scholar]
- 28.Ishimaru N, Arakaki R, Watanabe M, Kobayashi M, Miyazaki K, Hayashi Y. Development of autoimmune exocrinopathy resembling Sjogren's syndrome in estrogen-deficient mice of healthy background. Am J Pathol. 2003;163:1481–1490. doi: 10.1016/S0002-9440(10)63505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishimaru N, Arakaki R, Yoshida S, Yamada A, Noji S, Hayashi Y. Expression of the retinoblastoma protein RbAp48 in exocrine glands leads to Sjogren's syndrome-like autoimmune exocrinopathy. J Exp Med. 2008;205:2915–2927. doi: 10.1084/jem.20080174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishimaru N, Saegusa K, Yanagi K, Haneji N, Saito I, Hayashi Y. Estrogen deficiency accelerates autoimmune exocrinopathy in murine Sjogren's syndrome through fas-mediated apoptosis. Am J Pathol. 1999;155:173–181. doi: 10.1016/S0002-9440(10)65111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, Simpson ER. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci U S A. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konttinen YT, Fuellen G, Bing Y, Porola P, Stegaev V, Trokovic N, Falk SS, Liu Y, Szodoray P, Takakubo Y. Sex steroids in Sjögren's syndrome. J Autoimmun. 2012;39:49–56. doi: 10.1016/j.jaut.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Kuscu NK, Toprak AB, Vatansever S, Koyuncu FM, Guler C. Tear function changes of postmenopausal women in response to hormone replacement therapy. Maturitas. 2003;44:63–68. doi: 10.1016/s0378-5122(02)00316-x. [DOI] [PubMed] [Google Scholar]
- 34.Luu-The V, Labrie F. The intracrine sex steroid biosynthesis pathways. Prog Brain Res. 2010;181:177–192. doi: 10.1016/S0079-6123(08)81010-2. [DOI] [PubMed] [Google Scholar]
- 35.MacGillivray MH, Morishima A, Conte F, Grumbach M, Smith EP. Pediatric endocrinology update: an overview. The essential roles of estrogens in pubertal growth, epiphyseal fusion and bone turnover: lessons from mutations in the genes for aromatase and the estrogen receptor. Horm Res. 1998;49(Suppl 1):2–8. doi: 10.1159/000053061. [DOI] [PubMed] [Google Scholar]
- 36.McPherson SJ, Wang H, Jones ME, Pedersen J, Iismaa TP, Wreford N, Simpson ER, Risbridger GP. Elevated androgens and prolactin in aromatase-deficient mice cause enlargement, but not malignancy, of the prostate gland. Endocrinology. 2001;142:2458–2467. doi: 10.1210/endo.142.6.8079. [DOI] [PubMed] [Google Scholar]
- 37.Migeon BR. The role of X inactivation and cellular mosaicism in women's health and sex-specific diseases. Jama. 2006;295:1428–1433. doi: 10.1001/jama.295.12.1428. [DOI] [PubMed] [Google Scholar]
- 38.Mostafa S, Seamon V, Azzarolo AM. Influence of sex hormones and genetic predisposition in Sjogren's syndrome: a new clue to the immunopathogenesis of dry eye disease. Exp Eye Res. 2012;96:88–97. doi: 10.1016/j.exer.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson J, Steinberg A. Sex steroids, autoimmunity, and autoimmune diseases. In: Berczi I, Kovacs K, editors. Hormones and Immunity. MTP Press Limited; Lancaster, England: 1987. pp. 93–119. [Google Scholar]
- 40.Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocr Rev. 1996;17:369–384. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- 41.Ostrer H. Invited review: sex-based differences in gene expression. J Appl Physiol (1985) 2001;91:2384–2388. doi: 10.1152/jappl.2001.91.5.2384. [DOI] [PubMed] [Google Scholar]
- 42.Paliwal A, De PK. Marked sexual dimorphism of lacrimal gland peroxidase in hamster: repression by androgens and estrogens. Biochem Biophys Res Commun. 2006;341:1286–1293. doi: 10.1016/j.bbrc.2006.01.095. [DOI] [PubMed] [Google Scholar]
- 43.Pelit A, Bagis T, Kayaselcuk F, Dursun D, Akova Y, Aydin P. Tear function tests and conjunctival impression cytology before and after hormone replacement therapy in postmenopausal women. Eur J Ophthalmol. 2003;13:337–342. doi: 10.1177/112067210301300402. [DOI] [PubMed] [Google Scholar]
- 44.Rahimi Darabad R, Suzuki T, Richards SM, Jensen RV, Jakobiec FA, Zakka FR, Liu S, Sullivan DA. Influence of aromatase absence on the gene expression and histology of the mouse meibomian gland. Invest Ophthalmol Vis Sci. 2013;54:987–998. doi: 10.1167/iovs.12-10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richards SM, Jensen RV, Liu M, Sullivan BD, Lombardi MJ, Rowley P, Schirra F, Treister NS, Suzuki T, Steagall RJ, Yamagami H, Sullivan DA. Influence of sex on gene expression in the mouse lacrimal gland. Exp Eye Res. 2006;82:13–23. doi: 10.1016/j.exer.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 46.Rinn JL, Snyder M. Sexual dimorphism in mammalian gene expression. Trends Genet. 2005;21:298–305. doi: 10.1016/j.tig.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Roubinian JR, Talal N, Greenspan JS, Goodman JR, Siiteri PK. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J Exp Med. 1978;147:1568–1583. doi: 10.1084/jem.147.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sato EH, Sullivan DA. Comparative influence of steroid hormones and immunosuppressive agents on autoimmune expression in lacrimal glands of a female mouse model of Sjogren's syndrome. Invest Ophthalmol Vis Sci. 1994;35:2632–2642. [PubMed] [Google Scholar]
- 49.Schaumberg DA, Buring JE, Sullivan DA, Dana MR. Hormone replacement therapy and dry eye syndrome. Jama. 2001;286:2114–2119. doi: 10.1001/jama.286.17.2114. [DOI] [PubMed] [Google Scholar]
- 50.Seamon V, Vellala K, Zylberberg C, Ponamareva O, Azzarolo AM. Sex hormone regulation of tear lipocalin in the rabbit lacrimal gland. Exp Eye Res. 2008;87:184–190. doi: 10.1016/j.exer.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 51.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shim GJ, Warner M, Kim HJ, Andersson S, Liu L, Ekman J, Imamov O, Jones ME, Simpson ER, Gustafsson JA. Aromatase-deficient mice spontaneously develop a lymphoproliferative autoimmune disease resembling Sjogren's syndrome. Proc Natl Acad Sci U S A. 2004;101:12628–12633. doi: 10.1073/pnas.0405099101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simpson ER, Misso M, Hewitt KN, Hill RA, Boon WC, Jones ME, Kovacic A, Zhou J, Clyne CD. Estrogen--the good, the bad, and the unexpected. Endocr Rev. 2005;26:322–330. doi: 10.1210/er.2004-0020. [DOI] [PubMed] [Google Scholar]
- 54.Song X, Zhao P, Wang G, Zhao X. The effects of estrogen and androgen on tear secretion and matrix metalloproteinase-2 expression in lacrimal glands of ovariectomized rats. Invest Ophthalmol Vis Sci. 2014;55:745–751. doi: 10.1167/iovs.12-10457. [DOI] [PubMed] [Google Scholar]
- 55.Srikantan S, De PK. Sex differences in expression and differential regulation by androgen and estrogen of two odorant-binding tear lipocalins in lacrimal glands of immature hamsters. Gen Comp Endocrinol. 2008;158:268–276. doi: 10.1016/j.ygcen.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 56.Srikantan S, Paliwal A, Quintanar-Stephano A, De PK. Estrogen and androgen repression of two female specific lacrimal lipocalins in hamster: Pituitary independent and sex hormone receptor mediated action. Gen Comp Endocrinol. 2007;151:172–179. doi: 10.1016/j.ygcen.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 57.Sthoeger ZM, Zinger H, Mozes E. Beneficial effects of the anti-oestrogen tamoxifen on systemic lupus erythematosus of (NZBxNZW)F1 female mice are associated with specific reduction of IgG3 autoantibodies. Ann Rheum Dis. 2003;62:341–346. doi: 10.1136/ard.62.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sullivan DA. Tearful relationships? Sex, hormones, the lacrimal gland, and aqueous-deficient dry eye. Ocul Surf. 2004;2:92–123. doi: 10.1016/s1542-0124(12)70147-7. [DOI] [PubMed] [Google Scholar]
- 59.Sullivan DA, Jensen RV, Suzuki T, Richards SM. Do sex steroids exert sex-specific and/or opposite effects on gene expression in lacrimal and meibomian glands? Mol Vis. 2009;15:1553–1572. [PMC free article] [PubMed] [Google Scholar]
- 60.Suzuki T, Schirra F, Richards SM, Treister NS, Lombardi MJ, Rowley P, Jensen RV, Sullivan DA. Estrogen's and progesterone's impact on gene expression in the mouse lacrimal gland. Invest Ophthalmol Vis Sci. 2006;47:158–168. doi: 10.1167/iovs.05-1003. [DOI] [PubMed] [Google Scholar]
- 61.Takahashi M, Ishimaru N, Yanagi K, Haneji N, Saito I, Hayashi Y. High incidence of autoimmune dacryoadenitis in male non-obese diabetic (NOD) mice depending on sex steroid. Clin Exp Immunol. 1997;109:555–561. doi: 10.1046/j.1365-2249.1997.4691368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Talal N. Natural history of murine lupus. Modulation by sex hormones. Arthritis Rheum. 1978;21:S58–63. [PubMed] [Google Scholar]
- 63.Taner P, Akarsu C, Atasoy P, Bayram M, Ergin A. The effects of hormone replacement therapy on ocular surface and tear function tests in postmenopausal women. Ophthalmologica. 2004;218:257–259. doi: 10.1159/000078616. [DOI] [PubMed] [Google Scholar]
- 64.Toda I, Sullivan BD, Rocha EM, Da Silveira LA, Wickham LA, Sullivan DA. Impact of gender on exocrine gland inflammation in mouse models of Sjogren's syndrome. Exp Eye Res. 1999;69:355–366. doi: 10.1006/exer.1999.0715. [DOI] [PubMed] [Google Scholar]
- 65.Truong SS, Cole N, Stapleton F, Golebiowski B. Sex hormones and the dry eye. Clin Exp Optom. 2014;97:324–336. doi: 10.1111/cxo.12147. [DOI] [PubMed] [Google Scholar]
- 66.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 67.Wu WM, Lin BF, Su YC, Suen JL, Chiang BL. Tamoxifen decreases renal inflammation and alleviates disease severity in autoimmune NZB/W F1 mice. Scand J Immunol. 2000;52:393–400. doi: 10.1046/j.1365-3083.2000.00789.x. [DOI] [PubMed] [Google Scholar]
- 68.Xu J, Disteche CM. Sex differences in brain expression of X- and Y-linked genes. Brain Res. 2006;1126:50–55. doi: 10.1016/j.brainres.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 69.Zylberberg C, Seamon V, Ponomareva O, Vellala K, Deighan M, Azzarolo AM. Estrogen up-regulation of metalloproteinase-2 and -9 expression in rabbit lacrimal glands. Exp Eye Res. 2007;84:960–972. doi: 10.1016/j.exer.2007.02.002. [DOI] [PubMed] [Google Scholar]


