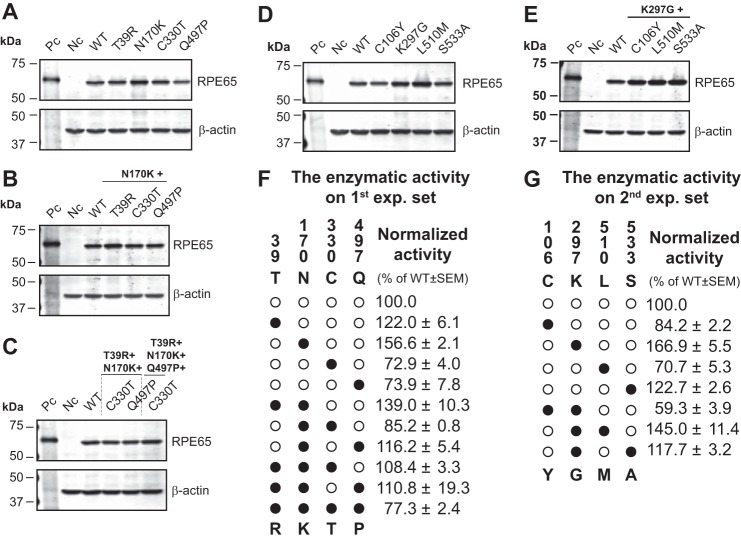
FIGURE 4.
Impacts of site-directed mutations on isomerohydrolase activity and protein level of RPE65. Plasmids expressing WT hRPE65 and cRPE65, and the indicated hRPE65 mutants were separately transfected into 293A-LRAT cells and cultured for 48 h. A–D, an equal amount of total proteins from cell lysates (20 μg) was used for Western blotting using an antibody specific for RPE65, with an anti-β-actin antibody as loading control (A, single; B, double; C, triple and quadruple mutants in the first experimental set; D, single; E, double mutants in the second experimental set. Pc, positive control (bovine RPE microsomal protein, 2.5 μg); Nc, negative control (red fluorescence protein); WT, WT hRPE65). RPE65 levels were semiquantified by densitometry and normalized by β-actin levels. F and G, in vitro activity assays were performed with the same batch of samples of Western blot analyses. Isomerohydrolase activities were quantified by generated 11-cis-[3H]retinol from three independent measurements and normalized by their relative RPE65 protein levels. Values were expressed as relative activity (percentage of WT hRPE65 activity, mean ± S.E., n = 3). In the enzymatic activity tables (F and G), human (○) and chicken (●) residues are indicated as white and black circles, respectively. The vertical numbers indicate the positions of candidate residues. Letters above and below the panel in the tables (F and G) represent hRPE65 and cRPE65 amino acid residues at the indicated positions, respectively (e.g. ●●○● in the table of the first experimental set (F) indicates a triple mutant (T39R/N170K/Q497P) of hRPE65).