FIGURE 7.
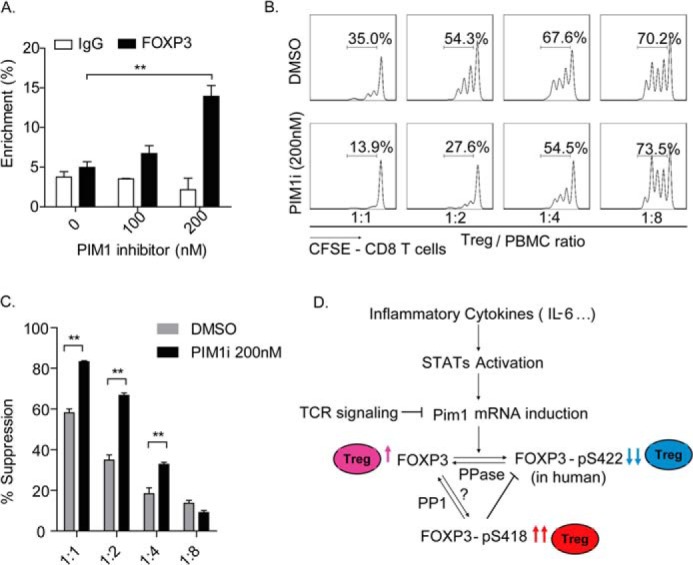
The PIM1-specific inhibitor enhances Treg suppressive function. A, in vitro expanded Tregs were treated with dimethyl sulfoxide (DMSO) or the PIM1-specific inhibitor at the indicated concentrations for 12 h. Samples were harvested for μChIP analysis, and the percent of enrichment of FOXP3 on the IL-2 promoter was detected with a specific anti-FOXP3 monoclonal antibody. **, p < 0.01. B, in vitro expanded human Tregs were pretreated with the PIM1-specific inhibitor (200 nm) or dimethyl sulfoxide for 2 days and then coincubated with CFSE-labeled PBMCs at the indicated ratios for an additional 3 days in the presence of the PIM1-specific inhibitor. CFSE dilution was determined for viable CD8+ T cells by flow cytometry. Data are from one representative experiment of three independent experiments with similar results. C, values indicate the percentages of suppression with mean ± S.E. of three independent experiments. **, p < 0.01. D, proposed working model for the mechanism of PIM1 regulation of human FOXP3 activity. Dephosphorylated human FOXP3 has normal standard DNA binding activity and confers immunosuppressive function to Tregs (pink). PIM1 could be induced by cytokines, including IL-6, through the STAT signaling pathway. Reversely, TCR signaling inhibits PIM1 mRNA transcription. The induced PIM1 phosphorylates human FOXP3 at its Ser422 site and negatively regulates its DNA binding activity, finally down-regulating the immunosuppressive function of Tregs (blue). On the other hand, phosphorylation at the Ser418 site of human FOXP3 would block phosphorylation of human FOXP3 at Ser422 by PIM1, therefore sustaining a high activity of human FOXP3 and the immunosuppressive function of Tregs (red).
