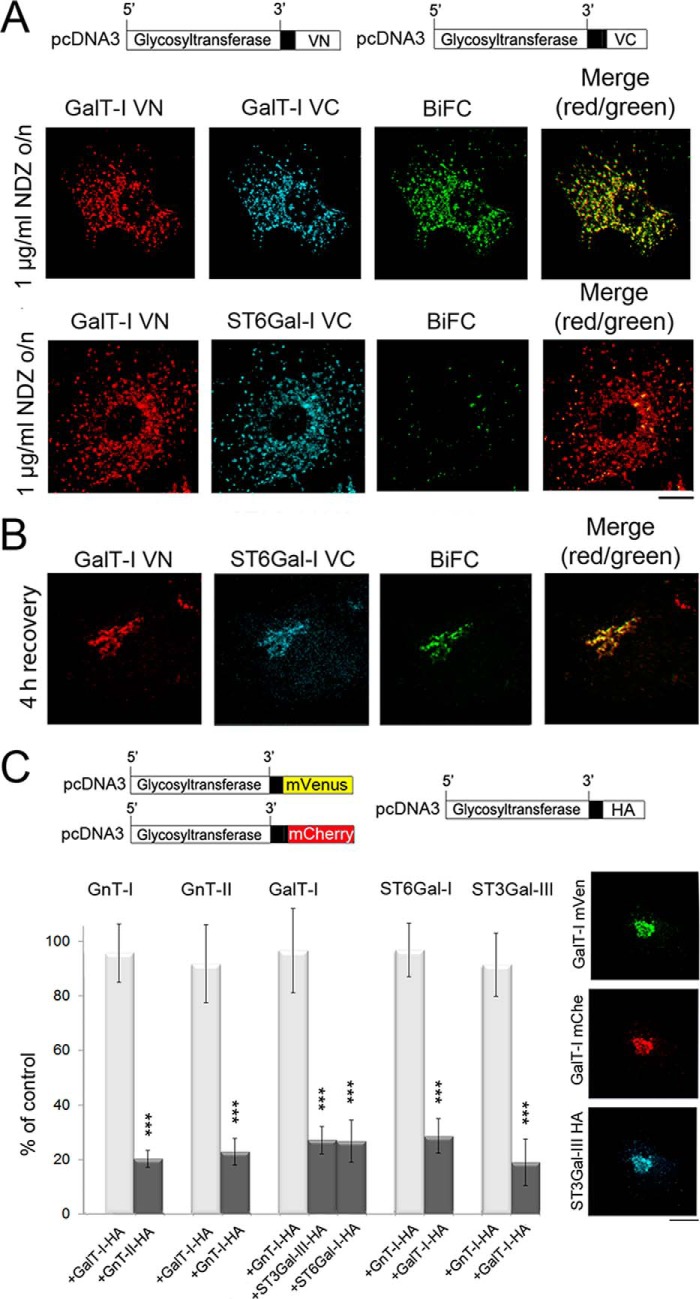
FIGURE 3.
Formation of glycosyltransferase heteromers in the Golgi after recovery from nocodazole treatment as detected by the BiFC approach. A, immunofluorescence microscopy of the cells transfected with the depicted homomeric and heteromeric GalT-I BiFC constructs. For transfections the plasmids without the N-terminal HA or FLAG tags were used. 8 h post-transfection cells were treated with 1 μg/ml nocodazole (NDZ) for 16 h and fixed before staining with the anti-GFP-VN- and anti-VC-specific antibodies. Note the localization of the enzyme constructs in the Golgi mini-stacks and the clear BiFC signal in the Golgi mini-stacks with the homomeric GalT-I constructs (top). In contrast, no BiFC signal was detected with the heteromeric GalT-I/ST6Gal-I constructs (bottom row). Scale bar, 10 μm. B, formation of GalT-I/ST6Gal-I heteromers after recovery from the nocodazole treatment. Cells treated as above were allowed to recover from nocodazole treatment for 4 h by removing the drug. Note the clear BiFC signal in the compact juxtanuclear Golgi structure. C, inhibition of homomer formation in the Golgi by the heteromeric enzyme constructs. In each case cells were transfected with the depicted homomeric FRET enzyme constructs (top) together with interacting (dark columns) or non-interacting (light columns) heteromeric enzyme constructs. Note that in each case only an interacting heteromeric enzyme construct significantly decreased the amount of homomers in the Golgi. The results are presented as percentages (the mean ± S.D., n = 10) of controls in which only the FRET constructs were used for the transfections. Fluorescence microscopy (right) shows the localization of the enzyme constructs in the Golgi of the transfected cells (***, p < 0.001).