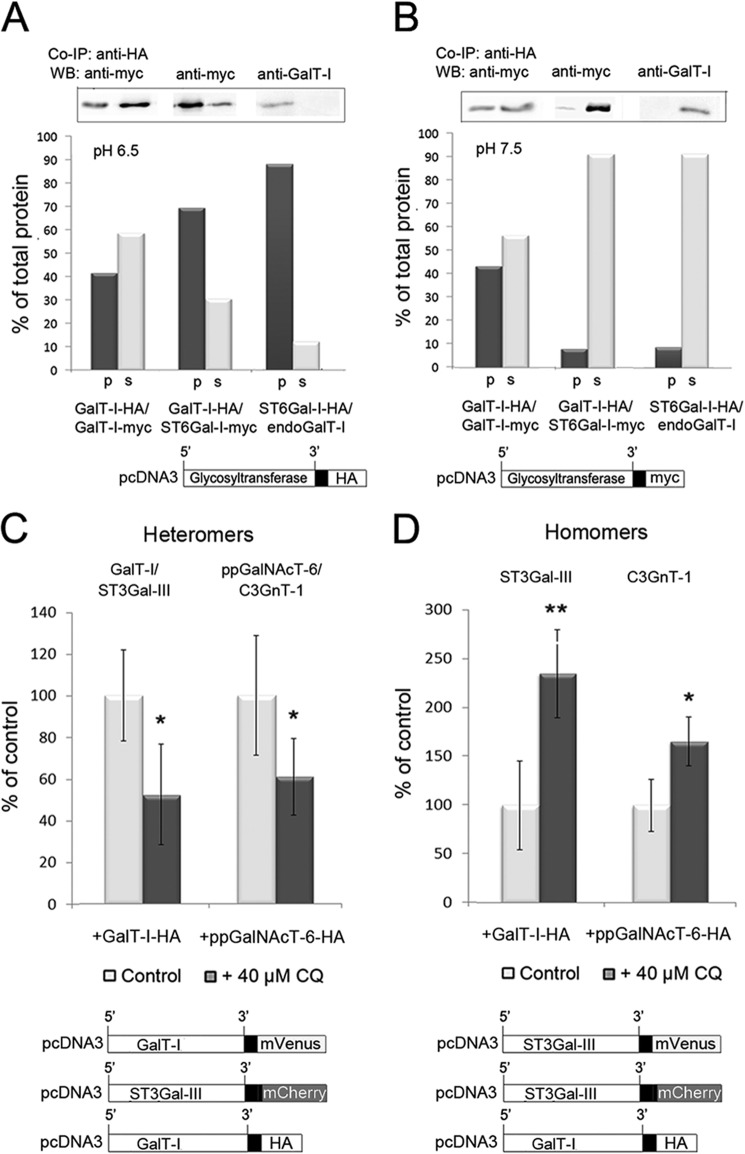
FIGURE 4.
The pH dependence of the Golgi-localized glycosyltransferase homomers and heteromers. A and B, co-immunoprecipitation (Co-IP) and Western blotting (WB) of GalT-I homomers or GalT-I/ST6Gal-I heteromers at pH 6.5 and 7.5. Cells were transfected with the depicted HA- and/or myc-tagged enzyme constructs, subjected to immunoprecipitation as described under “Experimental Procedures,” and immunoblotted from both the pellet (p) and supernatant (s) fractions with anti-myc or anti-GalT-I (for endogenous GalT-I) antibodies at both pH 6.5 (A) and pH 7.5 (B). At pH 6.5, note that both enzyme homomers and heteromers were recovered mainly in the pellet fractions, whereas at pH 7.5 >90% of the interacting enzymes were detected in the supernatant fractions. C and D, FRET microscopy quantification of the Golgi-localized enzyme heteromers (C) and homomers (D) in control and chloroquine-treated cells. Briefly, cells were transfected with the depicted (bottom) mVenus-, mCherry-, and HA-tagged enzyme constructs for 24 h before 40 μm CQ treatment. Note that the 0.4 pH unit increase in Golgi luminal pH induced by CQ markedly reduces the amount of enzyme heteromers in the Golgi (C), whereas it increases that of the enzyme homomers (D). The results are presented as percentages of non-treated controls (mean ± S.D., n = 10; *, p < 0.05; **, p < 0.01).