FIGURE 6.
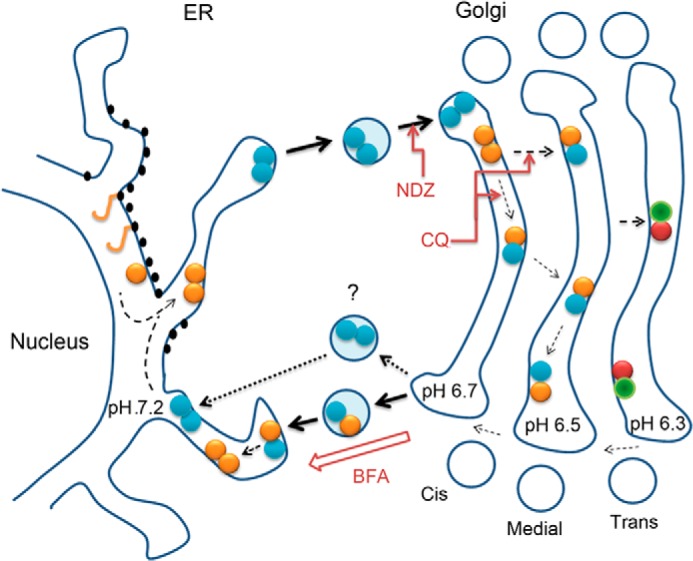
Schematic diagram of the organizational interplay between N-glycosyltransferase homo- and heteromers in live cells. After de novo synthesis, glycosyltransferase polypeptides (yellow sticks) form homodimers (double yellow or blue dots) during their folding and/or before their transport to the Golgi. In the Golgi acidic luminal milieu favors the formation of heteromers between sequentially acting medial-Golgi (yellow-blue dots) or trans-Golgi (red-green dots) enzymes at the expense of enzyme homomers. Nocodazole (NDZ) inhibits this process due to its ability to block anterograde transport and coalescence of transport vesicles into a compact Golgi structure. CQ, a pH gradient dissipating drug that impairs heteromer formation, was used to show that this transition is a pH-dependent process. Heteromers remain mobile in the Golgi membranes and are able to recycle to the ER. This was evidenced by using nocodazole-induced anterograde transport block that resulted in accumulation of the complexes in a more alkaline ER and a reverse transition from heteromers to enzyme homomers. Similar observations were obtained by using BFA that forces coalescence of Golgi membranes in the ER membranes. The question mark denotes a possibility that heteromer disassembly may already take place before the fusion of retrograde transport vesicles with the ER.
