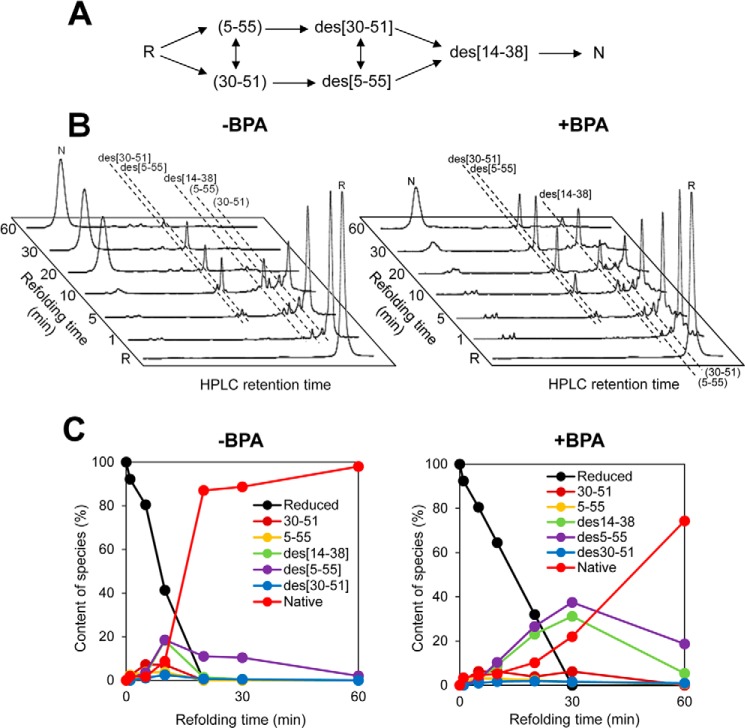
FIGURE 2.
Ero1α-PDI-driven oxidative folding of BPTI in the presence of BPA. A, schematic representation of the oxidative folding pathways of BPTI in the presence of glutathione. R and N represent reduced and native form of BPTI (5–55, 14–38, and 30–51). The disulfide parings of folding intermediates at each step are shown in parentheses, and the missing disulfide is indicated with the prefix “des.” B, HPLC profiles indicating the time course of oxidative folding of BPTI catalyzed by Ero1α-PDI in the absence (left) and presence (right) of BPA. Folding was initiated by the addition of Ero1α and PDI to reduced BPTI (30 μm) in a buffer saturated with molecular oxygen (no thiol agents, such as glutathione, were present). At the indicated time points, the reaction was quenched by HCl and analyzed by HPLC. C, quantitative analysis of each folding species based on the HPLC profiles shown in B. The occupancy of each species was plotted as a function of refolding time.