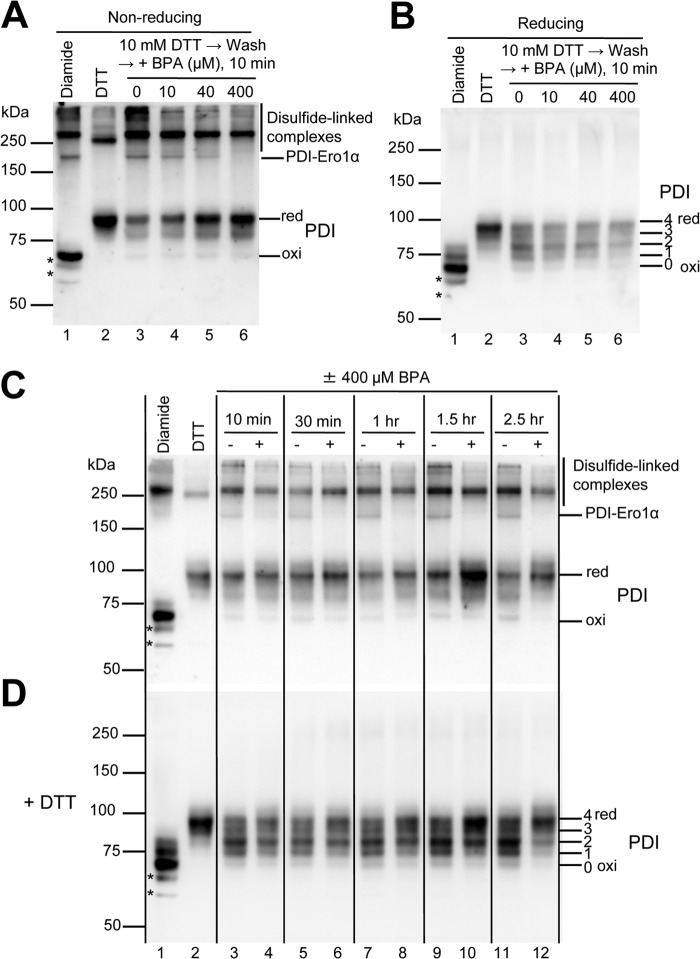
FIGURE 5.
BPA affects the redox state of PDI in HeLa cells. A and B, the effect of BPA on the reoxidation of PDI was studied by cultivating DTT-pretreated HeLa cells in fresh medium without DTT in the presence of the indicated concentrations of BPA for 10 min (lanes 3–6). To visualize the oxidation status of PDI, cellular proteins were subjected to alkylation with PEG-maleimide 2000 and separation on SDS-PAGE under non-reducing conditions in A and reducing conditions in B, followed by immunoblotting with antibody to PDI. Each lane contains 5 μg of proteins from each lysate. The positions of the disulfide-linked complexes that involve PDI are indicated on the right in A. The band assignable to PDI-Ero1α complex, which was also recognized by antibody to Ero1α (not shown), is marked as PDI-Ero1α. To know the positions of the fully oxidized (oxi) and reduced (red) forms of PDI, cells were treated with 1 mm diamide and 10 mm DTT for 10 min, respectively, before alkylation of free cysteines (lanes 1 and 2). Note that some disulfide-linked complexes are refractory to these treatments (see lanes 1 and 2 in A). The numbers of PEG-maleimide 2000 alkylation on the active site cysteines of PDI are indicated on the right in B. Note that the presence of PEG-maleimide 2000 in the sample caused slower migration of even the oxidized form of PDI on the gels when compared with PDI that was not treated with the alkylation reagent (not shown). We suggest that this could have been caused by the alkylation of two free non-catalytic cysteines on PDI (Cys312 and Cys343). Bands marked with an asterisk may represent some degradation products of PDI or nonspecific bands that appeared when the cultures were treated with diamide. C and D, to examine the effect of BPA on the redox state of PDI in cells, HeLa cells that had not been treated with DTT were incubated with or without 400 μm BPA for the indicated times. In lanes 3, 5, 7, and 9, dimethyl sulfoxide (a solvent of BPA) was added to the culture to a final concentration of 0.2%. To visualize the redox state of PDI, the cellular proteins were alkylated with PEG-maleimide 2000 as described in the legend to A. In D, proteins were reduced with 50 mm DTT before electrophoresis. Note that BPA caused the accumulation of a large portion of PDI in its reduced form, in agreement with the in vitro finding that BPA inhibited the Ero1α-catalyzed oxidation of PDI.