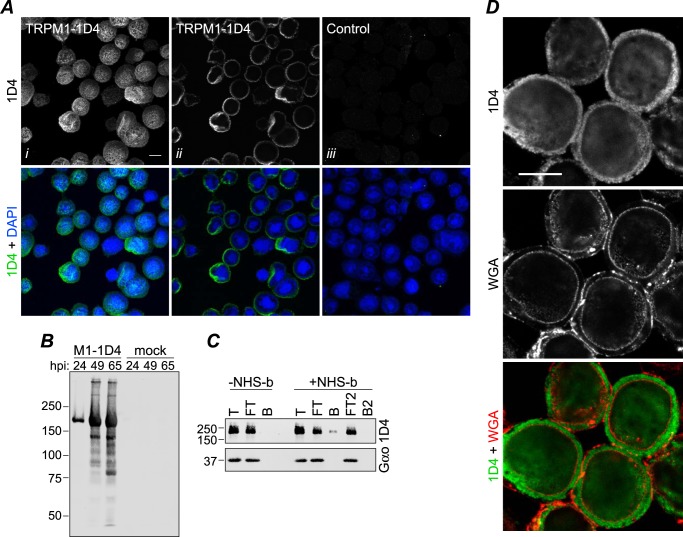
FIGURE 1.
Expression of TRPM1 in Sf9 cells. A, Sf9 cells were infected with baculovirus expressing TRPM1–1D4 (panels i and ii) or a control virus (GST-nyctalopin; panel iii). At 46 hpi, cells were fixed, permeabilized, and labeled with 1D4Ab (green) and DAPI (blue). Cells were imaged by confocal fluorescence microscopy; projections (panel i) or single optical slices near the middle of the cells (panels ii and iii) are shown. Scale bar, 10 μm. B, 1D4 Western blot of cells infected with TRPM1–1D4-expressing baculovirus or mock-infected and harvested at the indicated time point. C, live cells infected with baculovirus expressing TRPM1–1D4 or Gαo were treated with biotinylation reagent (+NHS-b) or mock-treated (−NHS-b) at ∼46 hpi. The reaction was quenched, cells were lysed in fos-choline-14, and biotinylated proteins were precipitated with streptavidin-agarose. Total (T), flow-through (FT), and bead-bound (B) fractions are shown. The flow-through was subjected to a second round of binding with fresh beads. No TRPM1 was detected in the second bound fraction (B2), indicating that the capacity of the beads was not exceeded. Similar results were obtained from two independent experiments. D, Sf9 cells infected with baculovirus expressing TRPM1–1D4 were labeled with WGA (red) at 42 hpi, then fixed, permeabilized, and labeled with 1D4Ab (green). A single optical slice is shown. Scale bar, 10 μm.