FIGURE 4.
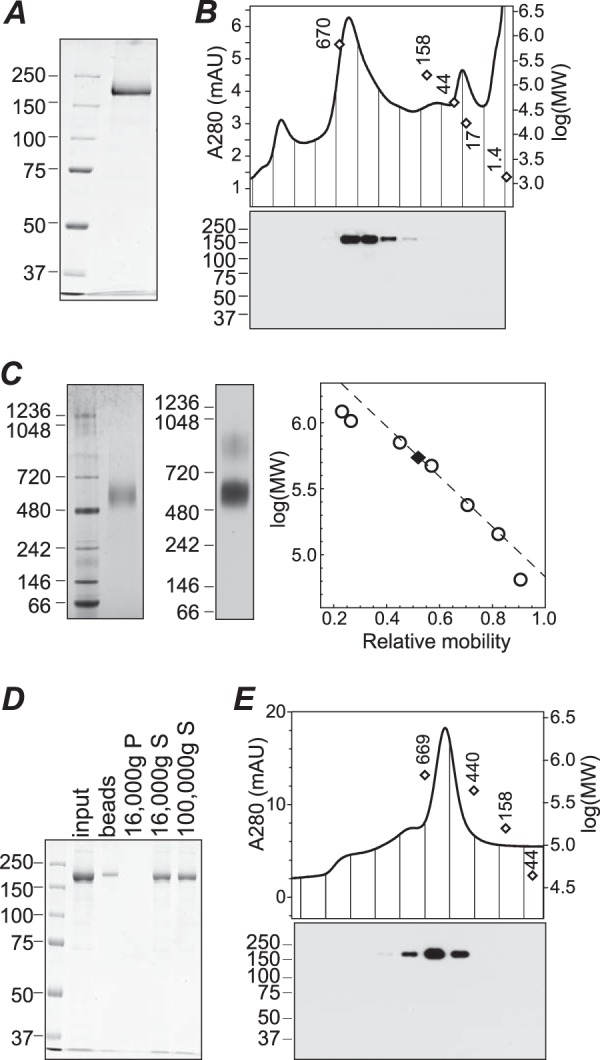
Purification of TRPM1 from Sf9 cell membranes. A, SDS-PAGE and Coomassie staining of purified protein. B, size exclusion chromatography of purified protein in buffer containing fos-choline-14. Fractions were subjected to SDS-PAGE and Western blotting (bottom). C, BN-PAGE of purified protein in fos-choline-14 followed by Coomassie staining (left) or 1D4 Western blotting (middle). The calibration curve used for determining the apparent molecular mass (right, dashed line) was constructed from the 720-, 480-, 242-, and 146-kDa markers. D, Coomassie-stained SDS-PAGE of input protein, protein stuck to BioBeads after amphipol exchange and detergent removal, and supernatant (S) and pellet (P) fractions after centrifugation at 16,000 × g. Approximately 50% of the input protein was recovered in the supernatant; further centrifugation at 100,000 × g did not result in additional loss of protein. E, size exclusion chromatography of TRPM1-amphipol complexes in detergent-free buffer. Fractions were subjected to 1D4 Western blotting (bottom) to confirm the identity of the peak. Peak positions of molecular weight markers in B and E are shown as open diamonds. mAU, milli-absorbance units.
