FIGURE 5.
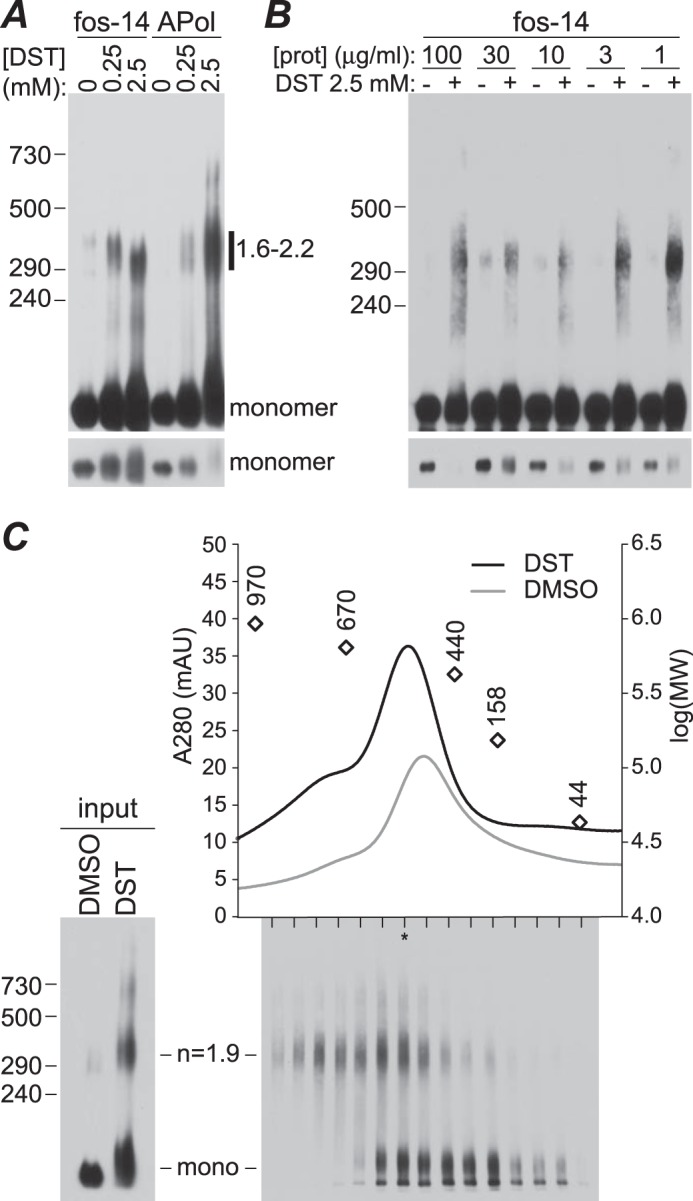
Cross-linking of purified TRPM1. A, purified protein in fos-choline-14 (fos-14) or complexed with amphipol (APol) was treated with 0.25 or 2.5 mm DST or mock-treated with DMSO and analyzed by SDS-PAAGE and 1D4 Western blotting. Calculated sizes of the cross-linked bands relative to the monomer molecular mass (184 kDa) are indicated. A shorter exposure of the same blot (bottom) is included to show the depletion of the monomer band in cross-linked samples. B, protein (prot) in fos-choline-14 was cross-linked at the indicated concentration and then diluted to equivalent concentrations for SDS-PAAGE and 1D4 Western blotting. C, size exclusion chromatography of cross-linked TRPM1. Protein-amphipol complexes cross-linked with 2.5 mm DST or mock-treated with DMSO were separated on a size exclusion chromatography column, and column fractions from the cross-linked sample were subjected to SDS-PAAGE and 1D4 Western blotting (bottom). The lane with the highest total protein, determined by quantification of total signal intensity in each lane, is indicated with an asterisk. Peak positions of molecular weight markers are shown as open diamonds. mAU, milli-absorbance units.
