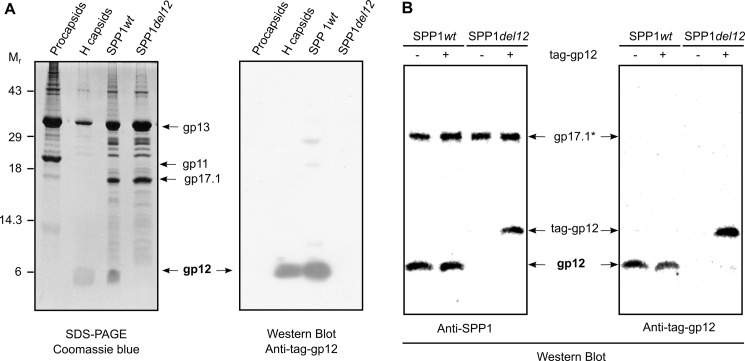
FIGURE 5.
Binding of gp12 to SPP1 capsids. A, composition of SPP1 assembly intermediates (cf. Fig. 1) determined by SDS-PAGE gel stained with Coomassie Blue (left panel) and presence of gp12 in the structures detected with anti-tag-gp12 polyclonal antibodies (right panel). The major capsid protein gp13, the major tail tube protein gp17.1, and the procapsid internal scaffolding protein gp11 are also identified in the Coomassie Blue-stained gel. B, binding of tag-gp12 to wild-type SPP1 and to SPP1del12 particles. Virions incubated overnight at 16 °C with tag-gp12, as indicated above the Western blot analyses, were separated from free protein by isopycnic centrifugation in cesium chloride gradients, and the composition of particles was analyzed by Western blotting with polyclonal antibodies raised against purified SPP1 virions (left panel) and anti-tag-gp12 antibodies (right panel). Note that gp12 and the tail protein gp17.1* (28) are the most immunogenic proteins of the SPP1 particle despite of the fact that they are not the most abundant components of the virion (A, left panel) (Refs. 28, 40 and this work).