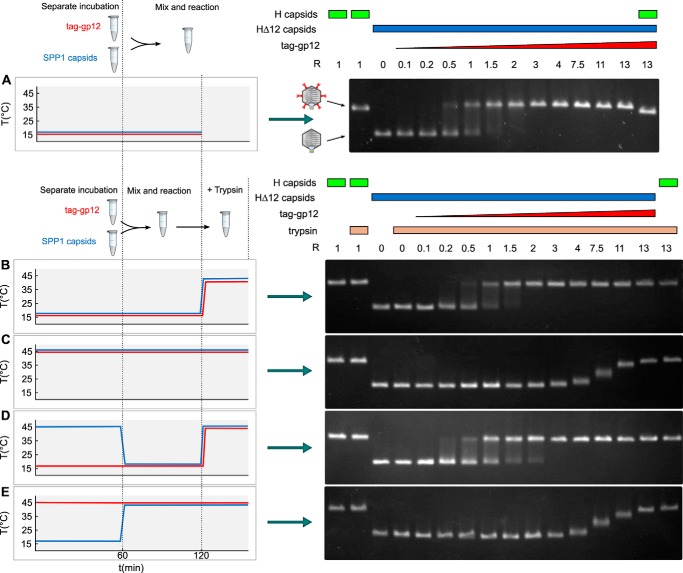
FIGURE 7.
Capsid binding behavior of native and unfolded tag-gp12. Purified SPP1 tailless capsids lacking gp12 (capsid HΔ12) (blue characters and blue curves on the left and blue rectangles above the gels on the right) and tag-gp12 (red) were preincubated separately, mixed, and treated with trypsin (except in A) according to the different combinations of incubation conditions used in the experiments in A–E (see “Results” for details), as outlined on the left of each panel. Samples treated with trypsin in B–E are identified by yellow rectangles above the gel lanes on the right. Capsids were then resolved by agarose gel electrophoresis to assess their occupancy with tag-gp12. Wild-type SPP1 capsids with gp12 (H capsids) (green rectangles above the gels on the right) and HΔ12 were used as controls. The schematics in the center of A show the electrophoretic mobility of capsid H (with gp12 represented in red) and HΔ12. The experiment was repeated four times independently.