FIGURE 8.
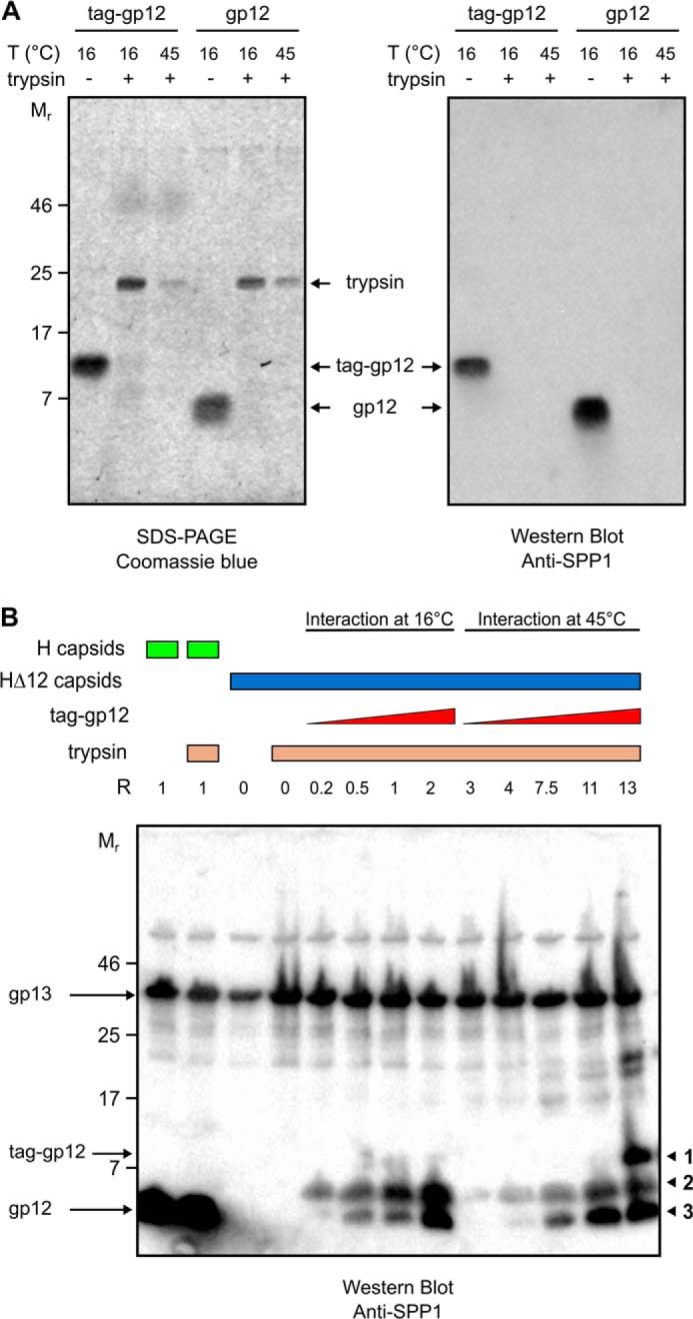
Trypsin sensitivity of free and capsid-bound gp12. A, purified tag-gp12 and gp12 were incubated with trypsin either at 16 or at 45 °C. Both proteins were completely digested by the protease at the tested temperatures, as assessed by Coomassie-stained SDS-PAGE (left panel) and Western blot analysis (right panel) with polyclonal anti-SPP1 antibodies that recognize gp12 (Fig. 5B). B, trypsin digestion of the binding reaction between capsids and tag-gp12 (labeled band 1 on the right of the figure) under the same conditions as in Fig. 7, B and C. Note that gp12 bound to H capsids is not sensitive to trypsin, whereas the tag of tag-gp12 is partially (band 2) or fully (band 3) digested by trypsin. The Western blot analysis was developed with anti-SPP1 antibodies that recognize gp12 but also, although giving a comparatively weak signal, the major capsid protein gp13, whose band was used to control the normalized input of capsids in the binding reaction.
