FIGURE 9.
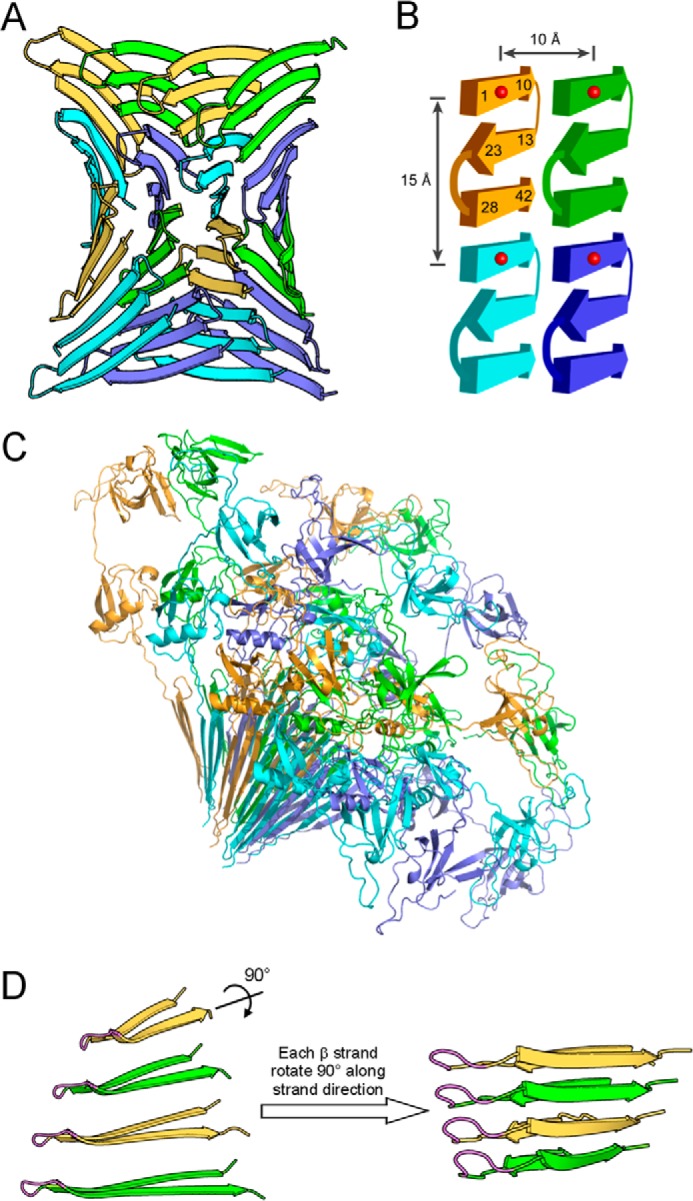
Atomic model of prefibrillar Aβ42 oligomers suggests a mechanism for oligomer-fibril interconversion. A, prefibrillar Aβ42 oligomers have a wrapped architecture wherein protofilament axes are correlated in phase with a central axis of 315 Å pitch. For clarity, Aβ subunits in oligomers are shown in four colors: green, yellow, slate blue, and cyan. B, each Aβ42 subunit consists of three β-strands that interact through backbone hydrogen bonds. The atomic model of panel A is schematized to illustrate measured distances at ∼10 and 15 Å. Numbers represent residue positions, and red balls represent spin labels. C, ribbon diagram of GU-Aβ42 oligomers with GroES and ubiquitin. D, strand rotation mechanism for oligomer-fibril interconversion. On the left, four Aβ42 monomers that interact through intersheet interaction are shown. These monomers are depicted as if looking down the β-sheet direction of the model in panel A. The N-terminal strand is omitted for clarity. The flexible turn identified by analysis of the inverse center line width (Fig. 4) is shown in magenta. Shown on the right is a fibril model of Aβ40 proposed previously (76). Prefibrillar oligomers may interconvert with fibril seeds by an ∼90° rotation around the long axes of the β-strands. We term this conversion mechanism as strand rotation.
