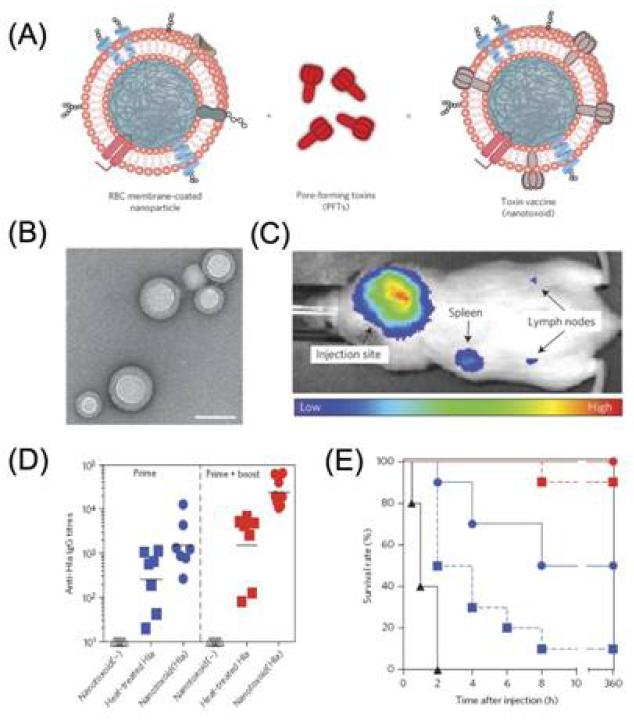
Figure 9.
(A) Schematic preparation of nanoparticle-detained toxins, consisting of substrate-supported RBC membranes into which PFTs can spontaneously incorporate.(B) TEM image of the particle vectors with uranyl-acetate staining (scale bar, 80 nm). (C) Live, whole-body fluorescent imaging of nanotoxoid(Hla) at 1h after subcutaneous administration. (D) Anti-Hla IgG titres at day 21 (n=7). Black lines indicate geometric means. Anti-Hla titres from mice vaccinated with non-toxin loaded particle vectors (nanotoxoid(-)) were monitored as controls (open triangles). (E) Unvaccinated mice (black triangles, solid line) and mice vaccinated with heat-treated Hla (prime; blue squares, dashed line), nanotoxoid(Hla) (prime; blue circles, solid line), heat-treated Hla (prime tboost; red squares, dashed line) or nanotoxoid(Hla) (prime + boost; red circle, solid line) received intravenous or subcutaneous administration of Hla. Survival rates of mice over a 15-day period following intravenous injections of 120 mg kg−1 Hla on day 21 via the tail vein (n=10). Reprinted with permission from [171]. Copyright 2013, Nature Publishing Group.