Fig. 2.
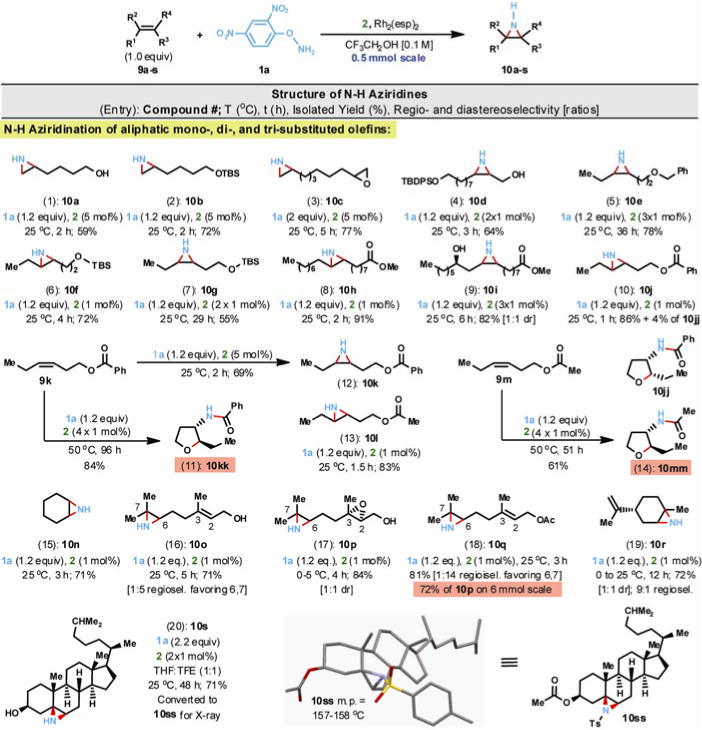
Direct and stereospecific N-H aziridination of olefins. Reactions were conducted at 0.1M using 2,2,2-trifluoroethanol as solvent and at 0.5 mmol scale unless otherwise indicated. To obtain crystalline material, 10s was O-acetylated and N-tosylated (Ts = para-toluenesulfonyl) to afford derivative 10ss.
