ABSTRACT
BACKGROUND
The relative contributions of depression, cognitive impairment without dementia (CIND), and dementia to the risk of potentially preventable hospitalizations in older adults are not well understood.
OBJECTIVE(S)
To determine if depression, CIND, and/or dementia are each independently associated with hospitalizations for ambulatory care-sensitive conditions (ACSCs) and rehospitalizations within 30 days after hospitalization for pneumonia, congestive heart failure (CHF), or myocardial infarction (MI).
DESIGN
Prospective cohort study.
PARTICIPANTS
Population-based sample of 7,031 Americans > 50 years old participating in the Health and Retirement Study (1998–2008).
MAIN MEASURES
The eight-item Center for Epidemiologic Studies Depression Scale and/or International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) depression diagnoses were used to identify baseline depression. The Modified Telephone Interview for Cognitive Status and/or ICD-9-CM dementia diagnoses were used to identify baseline CIND or dementia. Primary outcomes were time to hospitalization for an ACSC and presence of a hospitalization within 30 days after hospitalization for pneumonia, CHF, or MI.
KEY RESULTS
All five categories of baseline neuropsychiatric disorder status were independently associated with increased risk of hospitalization for an ACSC (depression alone: Hazard Ratio [HR]: 1.33, 95 % Confidence Interval [95%CI]: 1.18, 1.52; CIND alone: HR: 1.25, 95%CI: 1.10, 1.41; dementia alone: HR: 1.32, 95%CI: 1.12, 1.55; comorbid depression and CIND: HR: 1.43, 95%CI: 1.20, 1.69; comorbid depression and dementia: HR: 1.66, 95%CI: 1.38, 2.00). Depression (Odds Ratio [OR]: 1.37, 95%CI: 1.01, 1.84), comorbid depression and CIND (OR: 1.98, 95%CI: 1.40, 2.81), or comorbid depression and dementia (OR: 1.58, 95%CI: 1.06, 2.35) were independently associated with increased odds of rehospitalization within 30 days after hospitalization for pneumonia, CHF, or MI.
CONCLUSIONS
Depression, CIND, and dementia are each independently associated with potentially preventable hospitalizations in older Americans. Older adults with comorbid depression and cognitive impairment represent a particularly at-risk group that could benefit from targeted interventions.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-014-2916-8) contains supplementary material, which is available to authorized users.
KEY WORDS: depression, dementia, ambulatory care-sensitive condition, hospitalization, rehospitalization
INTRODUCTION
As the U.S. population ages, the burdens of chronic illnesses are growing,1,2 leading to increasing healthcare costs and concerns about Medicare’s sustainability.3,4 Hospitalizations among older adults remain costly for the American healthcare system,5 and one in five Medicare beneficiaries hospitalized for common conditions such as pneumonia, congestive heart failure (CHF), or myocardial infarction (MI) are rehospitalized within 30 days.5 Consequently, potentially preventable hospitalizations, such as those for ambulatory care-sensitive conditions (ACSCs) and early rehospitalizations, are of growing concern for healthcare providers and systems. To reduce hospitalization-related healthcare costs, the Centers for Medicare and Medicaid Services (CMS) is actively incentivizing efforts to reduce rehospitalizations within 30 days of initial hospitalization for pneumonia, CHF, or MI.6
Interest is increasing in understanding risk factors for potentially preventable hospitalizations and rehospitalizations among older adults.7–11 Although neuropsychiatric disorders such as depression, cognitive impairment without dementia (CIND), and dementia are highly prevalent in older adults,12–14 little is known about their potential contributions to preventable hospitalizations and rehospitalizations. The few prior studies examining the contribution of depression or dementia to the risk of these outcomes have been limited to single-centers,15,16 geographically defined health systems,17,18 or specific chronic disease populations.18 Furthermore, while depression is frequently comorbid with mild cognitive impairment and dementia,19 no study to date has considered the impact of comorbidity between depression and dementia on the risk of preventable hospitalizations and/or rehospitalizations.
The present investigation utilizes data from an ongoing longitudinal investigation of older Americans to determine if depression, CIND, and/or dementia are independently associated with increased risk of hospitalizations for ACSCs as well as rehospitalizations within 30 days of initial hospitalization for pneumonia, CHF, or MI. We hypothesized that depression, CIND, and dementia would each be independently associated with both hospitalization for an ACSC and 30-day rehospitalization.
METHODS
Population
Our study is a secondary analysis of prospectively collected, nationally representative data from U.S. adults over age 50 participating in the Health and Retirement Study (HRS). The HRS began in 1992. To date, over 31,000 individuals have participated. Subjects are interviewed every 2 years. The HRS follow-up rate has exceeded 90–95 % including proxies,20 and over 80 % of eligible respondents have consented to linkage of their Medicare claims records with study data. The HRS protocol was approved by the University of Michigan Institutional Review Board, and participants provided informed consent upon enrollment and again for linkage to Medicare claims.
Our sample included the 7,031 HRS respondents interviewed in 1998 or 2000 who consented to linkage of their Medicare claims records. We followed them through death or the 2008 interview.
Primary Independent Variable
The primary independent variable in our analyses was neuropsychiatric disorder status at baseline (i.e., the 1998 or 2000 HRS interview), defined categorically as no disorder, depression alone, CIND alone, dementia alone, comorbid depression and CIND, or comorbid depression and dementia.
Depression was defined as either a score of ≥ 4 on the eight-item Center for Epidemiologic Studies Depression Scale (CES-D)21 at the baseline HRS interview or a depression diagnosis in the Medicare claims in the year before baseline, based on International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) codes 296.2, 296.3, 298.0, 300.4, or 311.0. The eight-item CES-D cutoff score of ≥ 4 has been found to be comparable to the cutoff score of ≥ 16 on the full CES-D,21 and has been used in several prior studies.12,22,23
Cognitive impairment was assessed in the HRS using the modified Telephone Interview for Cognitive Status (TICSm).24 The TICSm has been validated against neuropsychiatric interview in the Aging, Demographics, and Memory Study, and has been found to have a weighted accuracy of 69.2 % in correct classification of individuals as having either normal cognition, CIND, or dementia.24 CIND was defined as a TICSm score of 7 to 11 at the baseline HRS interview.24 Dementia was defined as either a baseline TICSm score of ≤ 624 or a dementia diagnosis (ICD-9-CM codes 290.0-290.42, 291.2, 294.1, 294.8, 331.0, 331.1, 331.11, 331.19, or 331.82) in the Medicare claims in the year before baseline.
Covariates of Interest
Important covariates were chosen a priori based on prior research identifying their associations with health outcomes, including healthcare utilization, among older adults.5,7,10,11,14–18
Data on demographics (e.g., age, sex, race, education, marital/partnered status, and household net worth), alcohol use, and smoking came from the baseline HRS interview.
Clinical characteristics included chronic medical diagnoses used to compute a baseline Charlson Comorbidity score,25 and the number of hospitalizations in the year before baseline, both obtained from Medicare claims. Information on additional baseline health-risk behaviors was obtained from Medicare claims and included substance abuse (ICD-9-CM codes 303.0 – 305.0) as well as obesity (ICD-9-CM code 278.0) diagnoses.
Outcomes of Interest
Our two primary outcomes of interest were time to hospitalization for an ACSC and rehospitalization within 30 days after initial hospitalization for pneumonia, CHF, or MI. We used ICD-9-CM diagnostic codes to identify hospitalizations for which the principal discharge diagnosis was one of the conditions identified by the Agency for Healthcare Research and Quality (AHRQ) as ACSCs in their report on prevention quality indicators.26,27 Time to hospitalization for an ACSC was censored at death or the 2008 HRS interview. We identified acute hospitalizations that occurred within 30 days of initial hospitalization for pneumonia, CHF, or MI based on validated algorithms.28–30
Statistical Analysis
We present descriptive data as means and standard deviations (SDs) or proportions. To examine the association of baseline neuropsychiatric disorders with time to hospitalization for an ACSC, we used Cox proportional hazards regression models. First, we tested the unadjusted association of baseline neuropsychiatric disorder status with hospitalization for an ACSC. We then sequentially adjusted for our covariates of interest, categorizing all covariates that were not normally distributed. The sequence of adjustments was: 1) demographics (e.g., age, sex, race categorized as white versus non-white, education categorized as < high school graduate versus ≥ high school graduate, marital/partnered status categorized as married/partnered versus single/separated/widowed, household net worth categorized as ≥ the sample median net worth versus below); 2) clinical characteristics (e.g., Charlson score ≥ 1, ≥ 1 hospitalization in the year before baseline); and 3) health-risk behaviors (e.g., alcohol use categorized by the number of drinks per day, categorized smoking status, substance abuse and obesity diagnoses). We tested the proportional hazards assumption of each of the Cox models using Schoenfeld residuals and found that the fully adjusted model violated the proportional hazards assumption (P < 0.001 by chi-square test) due to age (P < 0.001 by chi-square test). Therefore, we repeated our fully adjusted proportional hazards regression including age as a time-varying covariate.31
We used logistic regression models with robust error variances to determine if baseline neuropsychiatric disorder status was independently associated with odds of rehospitalization within 30 days after initial hospitalization for pneumonia, CHF, or MI, using the same sequence of covariate adjustment as our models for ACSC-related hospitalizations.
To determine the extent that any increase in hospitalizations for ACSCs or 30-day rehospitalizations could be attributable to neuropsychiatric disorders, we calculated the population attributable fraction (PAF) for each baseline neuropsychiatric disorder. PAFs and their 95 % Confidence Intervals (95%CIs) were estimated using the following formulas: P(HR – 1) / (1 + P[HR – 1]) for ACSC-related hospitalizations, and P(OR – 1) / (1 + P[OR – 1]) for 30-day rehospitalizations. In these formulas, P represents the prevalence of the specific neuropsychiatric disorder, HR is the adjusted Hazard Ratio for the association of that neuropsychiatric disorder with hospitalization for an ACSC, and OR is the adjusted Odds Ratio for the association of the specific neuropsychiatric disorder with 30-day rehospitalization.32
We conducted three pre-specified sensitivity analyses. In the first sensitivity analysis, we examined whether there were competing risks for hospitalization for an ACSC, non-ACSC hospitalization, psychiatric hospitalization, or death using competing-risks regression models according to the Fine and Gray approach.33 Competing-risks regression models were implemented using stcrreg in STATA 12 (Stata Corporation, College Station, TX). In our second sensitivity analysis, we examined whether our models were influenced by adjusting for Elixhauser comorbidities34 rather than categorized Charlson score. In a third sensitivity analysis, we estimated the association of baseline neuropsychiatric disorder status with our outcomes of interest using only the eight-item CES-D and TICSm thresholds to define cases of depression or dementia. Since the eight-item CES-D was only administered to self-respondents,21 this sensitivity analysis only included the 6,256 eligible self-respondents.
We used two-sided significance tests for all analyses with statistical significance set at P = 0.05. Analyses were performed with appropriate components of the IBM SPSS Statistics 19 (SPSS Inc., Chicago, IL) and STATA 12 statistical software programs.
RESULTS
Table 1 describes the baseline demographic, clinical and health-risk behavioral characteristics of the total sample and grouped by baseline neuropsychiatric disorder status. At baseline, 12.0 % were depressed without substantial cognitive impairment, 13.4 % met the TICSm threshold for CIND without comorbid depression, 7.6 % had dementia without comorbid depression, 5.5 % had comorbid depression and CIND, and another 4.3 % had comorbid depression and dementia.
Table 1.
Sample Demographic and Clinical Characteristics at Baseline
| Entire cohort (n = 7,031) |
No depression, CIND, or dementia (n = 4,015) |
Depression alone (n = 842) |
CIND alone (n = 945) |
Dementia alone (n = 534) |
Depression and CIND (n = 390) |
Depression and dementia (n = 305) |
|
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age | 75.1 (8.3) | 73.8 (7.3) | 72.3 (9.2) | 77.3 (8.1) | 81.3 (8.3) | 75.7 (9.1) | 80.2 (8.5) |
| Sex | |||||||
| Male | 2,959 (42 %) | 1,853 (46 %) | 247 (29 %) | 422 (45 %) | 204 (38 %) | 128 (33 %) | 105 (34 %) |
| Female | 4,072 (58 %) | 2,162 (54 %) | 595 (71 %) | 523 (55 %) | 330 (62 %) | 262 (67 %) | 200 (66 %) |
| Race | |||||||
| White | 6,064 (86 %) | 3,649 (91 %) | 750 (89 %) | 778 (82 %) | 375 (70 %) | 283 (73 %) | 229 (75 %) |
| Non-white | 966 (14 %) | 365 (9 %) | 92 (11 %) | 167 (18 %) | 159 (30 %) | 107 (27 %) | 76 (25 %) |
| Education | |||||||
| ≥ High school graduate | 4,280 (61 %) | 2,839 (71 %) | 531 (63 %) | 459 (49 %) | 190 (36 %) | 160 (41 %) | 101 (33 %) |
| < High school graduate | 2,746 (39 %) | 1,171 (29 %) | 311 (37 %) | 486 (51 %) | 344 (64 %) | 230 (59 %) | 204 (67 %) |
| Marital status | |||||||
| Married/partnered | 3,996 (57 %) | 2,617 (65 %) | 406 (48 %) | 492 (52 %) | 208 (39 %) | 149 (38 %) | 124 (41 %) |
| Single/separated/widowed | 3,030 (43 %) | 1,397 (35 %) | 435 (52 %) | 453 (48 %) | 325 (61 %) | 240 (62 %) | 180 (59 %) |
| Net wortha | |||||||
| ≥ $125,200 | 3,514 (50 %) | 1,567 (39 %) | 458 (54 %) | 574 (61 %) | 383 (72 %) | 291 (75 %) | 241 (79 %) |
| < $125,200 | 3,517 (50 %) | 2,448 (61 %) | 384 (46 %) | 371 (39 %) | 151 (28 %) | 99 (25 %) | 64 (21 %) |
| Clinical characteristics | |||||||
| Charlson Score | 0.5 (1.3) | 0.4 (1.1) | 0.7 (1.3) | 0.5 (1.3) | 0.9 (1.6) | 0.9 (1.5) | 1.4 (1.7) |
| Myocardial infarction | 260 (4 %) | 118 (3 %) | 43 (5 %) | 39 (4 %) | 22 (4 %) | 22 (6 %) | 16 (5 %) |
| Congestive heart failure | 502 (7 %) | 173 (4 %) | 81 (10 %) | 66 (7 %) | 69 (13 %) | 51 (13 %) | 62 (20 %) |
| Peripheral vascular disease | 244 (3 %) | 103 (3 %) | 36 (4 %) | 27 (3 %) | 27 (5 %) | 24 (6 %) | 27 (9 %) |
| Cerebrovascular disease | 359 (5 %) | 153 (4 %) | 39 (5 %) | 37 (4 %) | 56 (10 %) | 30 (8 %) | 44 (14 %) |
| Chronic pulmonary disease | 558 (8 %) | 218 (5 %) | 104 (12 %) | 67 (7 %) | 56 (10 %) | 63 (16 %) | 50 (16 %) |
| Connective tissue disease | 63 (1 %) | 30 (1 %) | 11 (1 %) | 4 (0.4 %) | 6 (1 %) | 8 (2 %) | 4 (1 %) |
| Peptic ulcer disease | 99 (1 %) | 30 (1 %) | 18 (2 %) | 11 (1 %) | 15 (3 %) | 11 (3 %) | 14 (5 %) |
| Mild liver disease | 29 (0.4 %) | 9 (0.2 %) | 7 (1 %) | 3 (0.3 %) | 4 (1 %) | 1 (0.3 %) | 5 (2 %) |
| Diabetes w/o complications | 424 (6 %) | 174 (4 %) | 67 (8 %) | 57 (6 %) | 49 (9 %) | 38 (10 %) | 39 (13 %) |
| Diabetes w/ complications | 93 (1 %) | 37 (1 %) | 14 (2 %) | 8 (1 %) | 10 (2 %) | 12 (3 %) | 12 (4 %) |
| Paraplegia/hemiplegia | 79 (1 %) | 33 (1 %) | 12 (1 %) | 4 (0.4 %) | 11 (2 %) | 7 (2 %) | 12 (4 %) |
| Renal disease | 101 (1 %) | 34 (1 %) | 16 (2 %) | 15 (2 %) | 12 (2 %) | 12 (3 %) | 12 (4 %) |
| Cancer | 134 (2 %) | 77 (2 %) | 11 (1 %) | 12 (1 %) | 10 (2 %) | 11 (3 %) | 13 (4 %) |
| Moderate/severe liver disease | 5 (0.1 %) | 3 (0.1 %) | 1 (0.1 %) | 0 (0 %) | 0 (0 %) | 1 (0.3 %) | 0 (0 %) |
| Metastatic carcinoma | 59 (1 %) | 28 (1 %) | 8 (1 %) | 13 (1 %) | 7 (1 %) | 1 (0.3 %) | 2 (1 %) |
| AIDS/HIV | 0 (0 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) |
| ≥ 1 hospitalization in previous year | 1,438 (20 %) | 642 (16 %) | 210 (25 %) | 177 (19 %) | 147 (28 %) | 132 (34 %) | 130 (43 %) |
| # hospitalizations in previous year among hospitalized | 1.6 (1.1) | 1.6 (1.0) | 1.7 (1.1) | 1.6 (1.1) | 1.8 (1.2) | 1.7 (1.1) | 1.8 (1.2) |
| Health-risk behaviors | |||||||
| Alcohol use | |||||||
| Daily drinker | 1,664 (24 %) | 1,182 (29 %) | 147 (17 %) | 199 (21 %) | 58 (11 %) | 57 (15 %) | 21 (7 %) |
| # of drinks per day among daily drinkers | 1.8 (1.4) | 1.7 (1.3) | 1.8 (1.2) | 1.8 (1.7) | 1.9 (1.6) | 1.8 (1.3) | 2.4 (2.9) |
| Smoking status | |||||||
| Never smoked | 3,015 (43 %) | 1,664 (42 %) | 339 (40 %) | 440 (47 %) | 251 (48 %) | 172 (44 %) | 149 (49 %) |
| Former smoker | 3,197 (46 %) | 1,887 (47 %) | 386 (46 %) | 403 (43 %) | 242 (46 %) | 157 (41 %) | 122 (40 %) |
| Current smoker | 766 (11 %) | 434 (11 %) | 113 (14 %) | 96 (10 %) | 33 (6 %) | 59 (15 %) | 31 (10 %) |
| Substance abuse diagnosis | 87 (1 %) | 39 (1 %) | 18 (2 %) | 4 (0.4 %) | 5 (1 %) | 8 (2 %) | 13 (4 %) |
| Obesity | 91 (1 %) | 41 (1 %) | 17 (2 %) | 14 (1 %) | 5 (1 %) | 8 (2 %) | 6 (2 %) |
All values are N(%) or mean (SD)
Abbreviations (in alphabetical order): AIDS Acquired Immunodeficiency Syndrome; CIND cognitive impairment without dementia; HIV Human Immunodeficiency Virus
a The median household net worth is $125,200
Table 2 displays the number of hospitalizations for an ACSC among the entire sample and grouped by baseline neuropsychiatric disorder status. Over half of the hospitalizations for an ACSC during the follow-up period were among the 3,016 subjects with either depression, CIND, and/or dementia. There was no significant association between baseline neuropsychiatric disorder status and specific ACSC diagnosis (P = 0.52 by chi-square test).
Table 2.
Hospitalizations for Ambulatory Care-Sensitive Conditions Within Entire Sample and By Baseline Neuropsychiatric disorder status
| Entire cohort | No depression, CIND, or dementia | Depression alone | CIND alone | Dementia alone | Depression and CIND | Depression and dementia | |
|---|---|---|---|---|---|---|---|
| Total # of ACSC-related hospitalizations | 2,441 | 1,158 | 326 | 373 | 232 | 189 | 163 |
| ACSC-related hospitalizationsa | |||||||
| Admissions for diabetes with short-term complications | 16 (1 %) | 8 (1 %) | 2 (1 %) | 3 (1 %) | 0 (0 %) | 3 (2 %) | 0 (0 %) |
| Admissions for perforated appendix | 14 (0.4 %) | 10 (1 %) | 0 (0 %) | 4 (1 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) |
| Admissions for diabetes with long-term complications | 93 (4 %) | 43 (4 %) | 13 (4 %) | 13 (3 %) | 10 (4 %) | 7 (4 %) | 7 (4 %) |
| Admissions for chronic obstructive pulmonary disease | 255 (10 %) | 124 (11 %) | 43 (13 %) | 34 (9 %) | 17 (7 %) | 22 (12 %) | 15 (9 %) |
| Admissions for hypertension | 53 (2 %) | 30 (3 %) | 8 (2 %) | 8 (2 %) | 1 (0.4 %) | 2 (1 %) | 4 (2 %) |
| Admissions for congestive heart failure | 653 (27 %) | 306 (26 %) | 87 (27 %) | 114 (31 %) | 58 (25 %) | 52 (28 %) | 36 (22 %) |
| Admissions for dehydration | 342 (14 %) | 149 (13 %) | 45 (14 %) | 50 (13 %) | 36 (16 %) | 33 (17 %) | 29 (18 %) |
| Admissions for bacterial pneumonia | 607 (25 %) | 302 (26 %) | 71 (22 %) | 87 (23 %) | 65 (28 %) | 44 (23 %) | 38 (23 %) |
| Admissions for urinary tract infections | 273 (11 %) | 121 (10 %) | 39 (12 %) | 42 (11 %) | 35 (15 %) | 13 (7 %) | 23 (14 %) |
| Admissions for angina without concomitant procedures | 82 (3 %) | 42 (4 %) | 11 (3 %) | 12 (3 %) | 3 (1 %) | 8 (4 %) | 6 (4 %) |
| Admissions for uncontrolled diabetes without complications | 13 (0.5 %) | 5 (0.4 %) | 2 (1 %) | 1 (0.3 %) | 3 (1 %) | 1 (0.5 %) | 1 (1 %) |
| Admissions for lower extremity amputations among patients with diabetes | 26 (1 %) | 11 (1 %) | 3 (1 %) | 4 (1 %) | 4 (2 %) | 1 (0.5 %) | 3 (2 %) |
| Admissions for immunization-preventable influenza | 8 (0.3 %) | 5 (0.4 %) | 1 (0.3 %) | 0 (0 %) | 0 (0 %) | 2 (1 %) | 0 (0 %) |
| Admissions for immunization-preventable pneumococcal pneumonia | 6 (0.2 %) | 2 (0.2 %) | 1 (0.3 %) | 1 (0.3 %) | 0 (0 %) | 1 (0.5 %) | 1 (1 %) |
Abbreviations: ACSC ambulatory care-sensitive condition; CIND cognitive impairment without dementia
a There were no hospitalizations with a principal discharge diagnosis of adult asthma during the follow-up period
Associations of Baseline Neuropsychiatric Disorders with Hospitalization for an Ambulatory Care-Sensitive Condition
In unadjusted analyses, all five categories of baseline neuropsychiatric disorder status were associated with increased risk of hospitalization for an ACSC (depression alone: HR versus no disorder: 1.53, 95%CI: 1.35, 1.73; CIND alone: HR: 1.66, 95%CI: 1.48, 1.86; dementia alone: HR: 2.63, 95%CI: 2.28, 3.03; comorbid depression and CIND: HR: 2.38, 95%CI: 2.04, 2.77; comorbid depression and dementia: HR: 3.50, 95%CI: 2.95, 4.15).
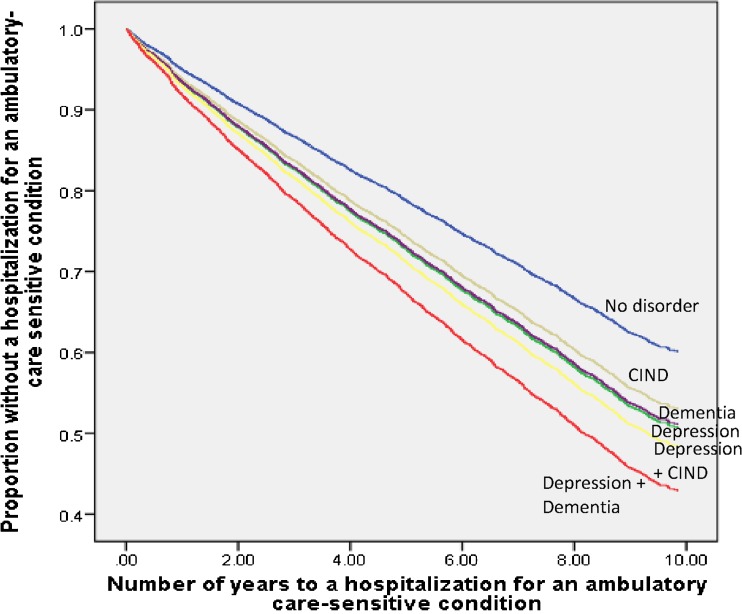
Table 3 displays the adjusted associations of baseline neuropsychiatric disorders with risk of hospitalization for an ACSC. All five categories of baseline neuropsychiatric disorder status were independently associated with increased risk of hospitalization for an ACSC relative to no disorder (depression alone: HR: 1.33, 95%CI: 1.18, 1.52; CIND alone: HR: 1.25, 95%CI: 1.10, 1.41; dementia alone: HR: 1.32, 95%CI: 1.12, 1.55; comorbid depression and CIND: HR: 1.43, 95%CI: 1.20, 1.69; comorbid depression and dementia: HR: 1.66, 95%CI: 1.38, 2.00), even after adjusting for demographics, clinical characteristics, and health-risk behaviors (Fig. 1). These associations remained unchanged by adjusting for age as a time-varying covariate (eTable 1 available online).
Table 3.
Adjusted Associations of Neuropsychiatric Disordersa at Baseline with Risk of Hospitalization for an Ambulatory Care-Sensitive Condition
| Adjusted for demographics | Adjusted for demographics and clinical characteristics | Adjusted for demographics, clinical characteristics and health-risk behaviors | |
|---|---|---|---|
| Hazard Ratio (95 % Confidence Interval) | |||
| Depression alone | 1.49 (1.31, 1.69)‡ | 1.38 (1.22, 1.57)‡ | 1.33 (1.18, 1.52)‡ |
| CIND alone | 1.24 (1.10, 1.40)† | 1.24 (1.10, 1.40)† | 1.25 (1.10, 1.41)‡ |
| Dementia alone | 1.53 (1.30, 1.79)‡ | 1.36 (1.16, 1.59)‡ | 1.32 (1.12, 1.55)† |
| Comorbid depression and CIND | 1.73 (1.47, 2.03)‡ | 1.48 (1.25, 1.74)‡ | 1.43 (1.20, 1.69)‡ |
| Comorbid depression and dementia | 2.15 (1.79, 2.57)‡ | 1.69 (1.41, 2.02)‡ | 1.66 (1.38, 2.00)‡ |
| Age | 1.04 (1.04, 1.05)‡ | 1.04 (1.04, 1.05)‡ | 1.05 (1.04, 1.05)‡ |
| Female | 0.78 (0.71, 0.85)‡ | 0.83 (0.75, 0.90)‡ | 0.89 (0.81, 0.98)* |
| Non-white | 1.03 (0.92, 1.16) | 1.08 (0.96, 1.22) | 1.07 (0.95, 1.21) |
| < High school graduate | 1.21 (1.10, 1.32)‡ | 1.16 (1.06, 1.27)† | 1.14 (1.04, 1.25)† |
| Single/separated/widowed | 1.19 (1.08, 1.30)‡ | 1.14 (1.04, 1.25)† | 1.11 (1.01, 1.22)* |
| Net worth < $125,000b | 1.54 (1.40, 1.69)‡ | 1.50 (1.36, 1.65)‡ | 1.44 (1.31, 1.59)‡ |
| Charlson score ≥ 1 | 2.19 (1.96, 2.45)‡ | 2.10 (1.87, 2.34)‡ | |
| ≥ 1 hospitalization in the previous year | 1.31 (1.17, 1.47)‡ | 1.31 (1.16, 1.47)‡ | |
| Alcohol consumption | |||
| 1 drink/day | 0.78 (0.68, 0.89)‡ | ||
| 2 drinks/day | 0.73 (0.60, 0.87)† | ||
| 3 drinks/day | 1.08 (0.80, 1.47) | ||
| ≥ 4 drinks/day | 0.98 (0.71, 1.34) | ||
| Smoking | |||
| Former smoker | 1.30 (1.19, 1.43)‡ | ||
| Current smoker | 1.65 (1.43, 1.90)‡ | ||
| Substance abuse diagnosis | 1.39 (0.99, 1.96) | ||
| Obesity | 1.37 (1.00, 1.88) | ||
Abbreviation: CIND cognitive impairment without dementia
* P < 0.05
† P < 0.01
‡ P < 0.001
a The comparison group for analyses of the association of neuropsychiatric disorders with risk of hospitalization for an ambulatory care-sensitive condition is those subjects with no disorder
b The median household net worth is $125,200
Figure 1.
Kaplan-Meier curve of fully adjusted model of years to a hospitalization for an ambulatory care-sensitive condition.
Associations of Baseline Neuropsychiatric Disorders with 30-Day Rehospitalizations
In unadjusted analyses, all five categories of baseline neuropsychiatric disorder status were associated with increased odds of rehospitalization within 30 days after initial hospitalization for pneumonia, CHF or MI (depression alone: OR: 1.58, 95%CI: 1.18, 2.11; CIND alone: OR: 1.53, 95%CI: 1.16, 2.03; dementia alone: OR: 1.82, 95%CI: 1.31, 2.54; comorbid depression and CIND: OR: 2.90, 95%CI: 2.10, 4.02; comorbid depression and dementia: OR: 2.78, 95%CI: 1.92, 3.99).
After adjusting for baseline demographic, clinical, and health-risk behavioral characteristics, depression alone (OR: 1.37, 95%CI: 1.01, 1.84), comorbid depression and CIND (OR: 1.98, 95%CI: 1.40, 2.81), or comorbid depression and dementia (OR: 1.58, 95%CI: 1.06, 2.35) at baseline were independently associated with increased odds of 30-day rehospitalization (Table 4).
Table 4.
Adjusted Associations of Neuropsychiatric Disordersa at Baseline with Odds of Rehospitalization Within 30 Days of an Initial Hospitalization for Pneumonia, Congestive Heart Failure, or Myocardial Infarction
| Adjusted for demographics | Adjusted for demographics and clinical characteristics | Adjusted for demographics, clinical characteristics and health-risk behaviors | |
|---|---|---|---|
| Odds Ratio (95 % Confidence Interval) | |||
| Depression alone | 1.58 (1.17, 2.12)† | 1.41 (1.05, 1.90)* | 1.37 (1.01, 1.84)* |
| CIND alone | 1.30 (0.98, 1.74) | 1.29 (0.97, 1.73) | 1.26 (0.94, 1.68) |
| Dementia alone | 1.37 (0.95, 1.96) | 1.19 (0.83, 1.59) | 1.18 (0.81, 1.70) |
| Comorbid depression and CIND | 2.39 (1.69, 3.38)‡ | 2.03 (1.43, 2.87)‡ | 1.98 (1.40, 2.81)‡ |
| Comorbid depression and dementia | 2.12 (1.43, 3.15)‡ | 1.59 (1.07, 2.38)* | 1.58 (1.06, 2.35)* |
| Age | 1.01 (1.00, 1.03)* | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.02) |
| Female | 0.74 (0.60, 0.91)† | 0.80 (0.63, 0.96)* | 0.79 (0.63, 0.99)* |
| Non-white | 1.11 (0.86, 1.44) | 1.15 (0.88, 1.49) | 1.15 (0.88, 1.50) |
| < High school graduate | 1.18 (0.96, 1.44) | 1.13 (0.92, 1.38) | 1.08 (0.88, 1.32) |
| Single/separated/widowed | 0.97 (0.77, 1.22) | 0.94 (0.75, 1.19) | 0.94 (0.74, 1.19) |
| Net worth < $125,000b | 1.52 (1.21, 1.91)‡ | 1.46 (1.16, 1.84)† | 1.45 (1.14, 1.84)† |
| Charlson score ≥ 1 | 2.01 (1.57, 2.57)‡ | 1.92 (1.49, 2.46)‡ | |
| ≥ 1 hospitalization in the previous year | 1.32 (1.03, 1.70)* | 1.33 (1.03, 1.72)* | |
| Alcohol consumption | |||
| 1 drink/day | 1.04 (0.76, 1.42) | ||
| 2 drinks/day | 0.38 (0.20, 0.70)† | ||
| 3 drinks/day | 0.56 (0.23, 1.39) | ||
| ≥ 4 drinks/day | 0.57 (0.23, 1.43) | ||
| Smoking | |||
| Former smoker | 1.21 (0.97, 1.51) | ||
| Current smoker | 1.06 (0.74, 1.51) | ||
| Substance abuse diagnosis | 1.04 (0.48, 2.24) | ||
| Obesity | 1.37 (0.74, 2.52) | ||
Abbreviation: CIND cognitive impairment without dementia
* P < 0.05
† P < 0.01
‡ P < 0.001
a The comparison group for analyses of the association of neuropsychiatric disorders with odds of 30-day rehospitalizations is those subjects with no disorder
b The median household net worth is $125,200.
Population Attributable Fraction Analyses
Table 5 displays the fractions of hospitalizations for ACSCs and 30-day rehospitalizations in our sample that are attributable to baseline neuropsychiatric disorders. In our sample, 14 % of the hospitalizations for an ACSC and 15 % of the 30-day rehospitalizations were attributable to baseline neuropsychiatric disorders.
Table 5.
Population Attributable Fractions of Hospitalizations for Ambulatory Care-Sensitive Conditions and 30-day Rehospitalizations Attributable to Neuropsychiatric Disorders Versus No Disorder
| Hospitalizations for an ACSC | 30-day Rehospitalizations | |
|---|---|---|
| Population attributable fraction (95 % Confidence Interval) | ||
| Depression alone | 4 % (2 %, 6 %) | 4 % (0.1 %, 9 %) |
| CIND alone | 3 % (1 %, 5 %) | 3 % (-1 %, 8 %) |
| Dementia alone | 2 % (1 %, 4 %) | 1 % (-1 %, 5 %) |
| Comorbid depression and CIND | 2 % (1 %, 4 %) | 5 % (2 %, 9 %) |
| Comorbid depression and dementia | 3 % (2 %, 4 %) | 2 % (0.3 %, 5 %) |
Abbreviations: ACSC ambulatory care-sensitive condition; CIND cognitive impairment without dementia
Sensitivity Analyses
In our competing-risks regression sensitivity analyses, the baseline presence of depression alone, CIND alone, comorbid depression and CIND, or comorbid depression with dementia were all independently associated with increased risk of hospitalization for an ACSC when accounting for the competing risks of non-ACSC hospitalization (n = 3,093), psychiatric hospitalization (n = 67), or death (n = 409) (eTable 2 available online).
Adjusting for non-psychiatric Elixhauser comorbidities instead of categorized Charlson score did not substantively impact our analyses of the association of baseline neuropsychiatric disorder status with hospitalization for an ACSC (eTable 3 and eFigure 1 available online). Comorbidity between depression and CIND or depression and dementia at baseline remained independently associated with increased odds of rehospitalization within 30 days after hospitalization for pneumonia, CHF, or MI when adjusting for non-psychiatric Elixhauser comorbidities rather than categorized Charlson score (eTable 3 available online).
Defining baseline depression only by an eight-item CES-D score ≥ 4 and baseline dementia only by a TICSm score ≤ 6 did not impact the analyses of the association of baseline neuropsychiatric disorder status with hospitalization for an ACSC (eTable 4 and eFigure 2 available online), while comorbidity between depression and CIND or depression and dementia both remained independently associated with increased odds of 30-day rehospitalization (eTable 4 available online).
DISCUSSION
In this nationwide sample of older Americans, we have identified that neuropsychiatric disorders are important contributors to potentially preventable hospitalizations and rehospitalizations. All three neuropsychiatric disorders were independently associated with increased risk of hospitalization for an ACSC, and depression as well as comorbidity between depression and CIND or depression and dementia were independently associated with rehospitalization within 30 days after initial hospitalization for pneumonia, CHF, or MI. Our findings build upon prior studies identifying that older adults with comorbid depression and cognitive impairment are a uniquely at-risk population for a wide range of adverse outcomes, including institutionalization, mortality, and caregiver burden.35,36
The potential implications of identifying that depression, CIND, and dementia each have independent associations with increased risk of preventable hospitalizations and early rehospitalizations are of public health importance. An emerging literature has found that hospitalizations among older adults for a wide range of medical illnesses may increase the risk of cognitive and functional decline as well as depression.37–40 Therefore, our findings suggest the potential for a vicious cycle of hospitalization, rehospitalization, and precipitous decline among older adults with pre-existing depression and/or cognitive impairment.
Depression, CIND, and dementia could increase the risk of preventable hospitalizations and rehospitalizations by several mechanisms. Depression, CIND, and dementia can lead to non-adherence with treatment for chronic conditions and difficulty with care coordination.17,41 Depression is also associated with increased systemic inflammation as well as hypothalamic pituitary axis and autonomic nervous system dysregulation,41,42 further potentiating the development of medical-surgical complications.
In addition to neuropsychiatric disorders and other previously identified risk factors such as increased age,5,11,18 greater medical comorbidity,5,18 and prior hospitalization,5,18 we found that lower household net worth at baseline, an indicator of lower socioeconomic status, was independently associated with both increased risk of hospitalization for an ACSC as well as 30-day rehospitalization. These findings expand upon prior work that has identified lower socioeconomic status as a potential risk factor for potentially preventable hospitalizations and rehospitalizations.5,43 We also found that mild-to-moderate daily alcohol consumption among our sample was independently associated with decreased risk of both hospitalization for an ACSC and 30-day rehospitalization, which could be partially explained by the potential benefit of mild-to-moderate alcohol use for cardiovascular health or functioning in older adults.44,45
An implication of identifying that depression, whether alone or comorbid with CIND or dementia, could raise the risk of potentially preventable hospitalizations and rehospitalizations, is that it is modifiable. Several primary care-based interventions have reduced depressive symptoms in older adults,46–49 and have also been adapted to improve chronic medical illness management.49 Similar interventions have also decreased neuropsychiatric symptoms and behavioral disturbances in older adults with dementia.50 Additional research is needed to examine if aspects of these interventions could be adapted to acute care settings to enhance existing interventions that target improving care transitions from the hospital back to primary care,51 particularly since CMS has invested heavily in piloting programs to prevent early rehospitalizations.52
Our study has several limitations. While ACSCs are an important quality indicator, it remains unclear to what extent enhanced quality of ambulatory care could prevent these hospitalizations, necessitating caution when considering the implications of our findings. Since we only assessed neuropsychiatric disorder status at baseline, and depression status may have changed over the course of follow-up, it is reasonable to consider that other more proximate factors may have played a greater role in hospitalization for an ACSC or 30-day rehospitalization. However, prior work has established that depression in older adults with medical illnesses is frequently chronic,47 and given the progressive nature of dementia, it is unlikely that its association with these outcomes would decrease over time. Furthermore, the probable effect of dementia as a risk factor for hospitalization for an ACSC or 30-day rehospitalization is likely greater than presented here, since 10–20 % of older adults with CIND progress to dementia annually.13
We acknowledge that our assessment of baseline neuropsychiatric disorder status could be subject to misclassification bias, since diagnosing depression in older adults with dementia is difficult and cognitive impairment can be due to depression itself. This possibility may be increased by inclusion of ICD-9-CM codes for dementia with depressive features in our dementia definition. Our definition of baseline neuropsychiatric disorder status also precludes consideration of neuropsychiatric symptom severity as a potential mediator. Although the eight-item CES-D has not been specifically validated in cognitively impaired older adults, and the TICSm has not been specifically validated in depressed older adults, both have been used previously in other relevant studies of older adults with high rates of these disorders.12,14,22,23,38,40 An additional limitation is that baseline factors such as alcohol use or smoking may not accurately reflect subsequent health behaviors over the follow-up period. Also, adjustment for ICD-9-CM obesity diagnosis likely underestimated the impact of this important confounder. Furthermore, we do not have data on neuropsychiatric disorder treatments in our cohort to be able to infer whether appropriate therapies could modify the associations presented here. Finally, residual confounding remains a possibility, as in any observational study.
In conclusion, using a nationwide sample of older Americans, we have shown that depression, CIND, and dementia are each independently associated with increased risk of hospitalization for an ACSC and rehospitalization within 30 days after hospitalization for pneumonia, CHF, or MI. In addition, we identified that older adults with comorbidity between depression and CIND or depression and dementia are at greatest risk for these potentially preventable adverse outcomes. Additional research that furthers understanding of the bi-directional relationship between neuropsychiatric disorders such as depression, CIND, and dementia, with hospitalizations, as well as develops strategies for targeting interventions to reduce hospitalizations in this high risk group, is necessary in order to help maintain quality of life and independent functioning in older adults.
Electronic supplementary material
(DOCX 89 kb)
(DOCX 18 kb)
(DOCX 20 kb)
(DOCX 18 kb)
(DOCX 51 kb)
(DOCX 48 kb)
Acknowlegdements
The Health and Retirement Study was performed at the Institute for Social Research, University of Michigan. We appreciate the expert programming of Laetitia Shapiro at the University of Michigan.
FUNDING
This work was supported by grants KL2 TR000421, K08 HL091249, R01 AG030155, and U01 AG09740 from the National Institutes of Health.
POTENTIAL CONFLICTS OF INTEREST
Dr. Katon has received honorariums for CME lectures from Eli Lilly, Forest, and Pfizer pharmaceutical companies. Drs. Davydow, Zivin, Pontone, Chwastiak, Langa, and Iwashyna have no relevant potential conflicts of interest to disclose.
DISCLAIMER
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs, the National Institutes of Health, or the US government.
Footnotes
Dr. Davydow has had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
REFERENCES
- 1.Aronow WS. Heart disease and aging. Med Clin North Am. 2006;90:849–862. doi: 10.1016/j.mcna.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Caspersen CJ, Thomas GD, Boseman LA, Beckles GLA, Albright AL. Aging, diabetes, and the public health system in the United States. Am J Public Health. 2012;102:1482–1497. doi: 10.2105/AJPH.2011.300616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuehn BM. Cutting Medicare costs will require multipronged approach. JAMA. 2013;309:1334–1335. doi: 10.1001/jama.2013.2771. [DOI] [PubMed] [Google Scholar]
- 5.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Medicare and Medicaid Services. Readmissions Reduction Program. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html Accessed May 23, 2014.
- 7.Niefeld MR, Braunstein JB, Wu AW, Saudek CD, Weller WE, Anderson GF. Preventable hospitalizations among elderly Medicare beneficiaries with type 2 diabetes. Diabetes Care. 2003;26:1344–1349. doi: 10.2337/diacare.26.5.1344. [DOI] [PubMed] [Google Scholar]
- 8.Fry AM, Shay DK, Holman RC, Curns AT, Anderson LJ. Trends in hospitalization for pneumonia among persons aged 65 years or older in the United States, 1988-2002. JAMA. 2005;294:2712–2719. doi: 10.1001/jama.294.21.2712. [DOI] [PubMed] [Google Scholar]
- 9.Yan LL, Daviglus ML, Liu K, et al. Midlife body mass index and hospitalization and mortality in older age. JAMA. 2006;295:190–198. doi: 10.1001/jama.295.2.190. [DOI] [PubMed] [Google Scholar]
- 10.Robinson S, Howie-Esquivel J, Vlahov D. Readmission risk factors after hospital discharge among the elderly. Popul Health Manag. 2012;15:338–351. doi: 10.1089/pop.2011.0095. [DOI] [PubMed] [Google Scholar]
- 11.Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalizations for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355–363. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zivin K, Pirraglia PA, McCammon RJ, Langa KM, Vijan S. Trends in Depressive Symptom Burden Among Older Adults in the United States from 1998 to 2008. J Gen Intern Med. 2013;28:1611–1619. doi: 10.1007/s11606-013-2533-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148:427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368:1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell SE, Paasche-Orlow MK, Forsythe SR, et al. Post-discharge hospital utilization among adult medical inpatients with depressive symptoms. J Hosp Med. 2010;5:378–384. doi: 10.1002/jhm.673. [DOI] [PubMed] [Google Scholar]
- 16.Burke RE, Donzé J, Schnipper JL. Contribution of psychiatric illness and substance abuse to 30-day readmission risk. J Hosp Med. 2013;8:450–455. doi: 10.1002/jhm.2044. [DOI] [PubMed] [Google Scholar]
- 17.Phelan EA, Borson S, Grothaus L, Balch S, Larson EB. Association of incident dementia with hospitalizations. JAMA. 2012;307:165–172. doi: 10.1001/jama.2011.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davydow DS, Katon WJ, Lin EHB, et al. Depression and risk of hospitalizations for ambulatory care-sensitive conditions in patients with diabetes. J Gen Intern Med. 2013;28:921–929. doi: 10.1007/s11606-013-2336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the Cardiovascular Health Study. JAMA. 2002;288:1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 20.Health and Retirement Study: Sample sizes and response rates. http://hrsonline.isr.umich.edu/sitedocs/sampleresponse.pdf. Accessed May 22, 2014.
- 21.Steffick DE. Documentation of affective functioning measures in the Health and Retirement Study. Ann Arbor, Michigan: Survey Research Center; 2000. [Google Scholar]
- 22.Langa KM, Valenstein MA, Fendrick AM, Kabeto MA, Vijan S. Extent and cost of informal caregiving for older Americans with symptoms of depression. Am J Psychiatry. 2004;161:857–863. doi: 10.1176/appi.ajp.161.5.857. [DOI] [PubMed] [Google Scholar]
- 23.Zivin K, Llewellyn DJ, Lang IA, et al. Depression among older adults in the United States and England. Am J Geriatr Psychiatr. 2010;18:1036–1044. doi: 10.1097/JGP.0b013e3181dba6d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: the health and retirement study and the aging, demographics, and memory study. J Gerontol B Psychol Sci Soc Sci. 2011;66(Suppl 1):i162–i171. doi: 10.1093/geronb/gbr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL. A new method for classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 26.Agency for Healthcare Research and Quality. AHRQ Quality Indicators - Guide to Prevention Quality Indicators: Hospital Admission for Ambulatory Care Sensitive Conditions. Rockville, MD: Agency for Healthcare Research and Quality, 2001. AHRQ Pub No. 02-R0203.
- 27.Coffey R, Barrett M, Houchens R, et al. Methods Applying AHRQ Quality Indicators to Healthcare Cost and Utilization Project (HCUP) Data for the Seventh (2009) National Healthcare Quality Report. HCUP Methods Series Report #2009-01. Rockville, MD: Agency for Healthcare Research and Quality. August 2009.
- 28.Aronsky D, Haug PJ, Lagor C, et al. Accuracy of administrative data for identifying patients with pneumonia. Am J Med Qual. 2005;20:319–328. doi: 10.1177/1062860605280358. [DOI] [PubMed] [Google Scholar]
- 29.Saczynski JS, Andrade SE, Harrold LR, et al. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):129–140. doi: 10.1002/pds.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148:99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Allison PD. Survival analysis. http://www.statisticalhorizons.com/wp-content/uploads/2012/01/Allison_SurvivalAnalysis.pdf. Accessed May 22, 2014.
- 32.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88:15–19. doi: 10.2105/AJPH.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 34.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Okura T, Plassman BL, Steffens DC, Llewellyn DJ, Potter GG, Langa KM. Neuropsychiatric symptoms and the risk of institutionalization and death: the Aging, Demographics, and Memory Study. J Am Geriatr Soc. 2011;59:473–481. doi: 10.1111/j.1532-5415.2011.03314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okura T, Langa KM. Caregiver burden and neuropsychiatric symptoms in older adults with cognitive impairment: the Aging, Demographics, and Memory Study. Alzheimer Dis Assoc Disord. 2011;25:116–121. doi: 10.1097/WAD.0b013e318203f208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehlenbach WJ, Hough CL, Crane PK, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303:763–770. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson RS, Hebert LE, Scherr PA, Dong X, Leurgens SE, Evans DA. Cognitive decline after hospitalization in a community population of older persons. Neurology. 2012;78:950–956. doi: 10.1212/WNL.0b013e31824d5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davydow DS, Hough CL, Levine DA, Langa KM, Iwashyna TJ. Functional disability, cognitive impairment, and depression after hospitalization for pneumonia. Am J Med. 2013;126:615–624. doi: 10.1016/j.amjmed.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol Psychiatry. 2003;54:216–226. doi: 10.1016/S0006-3223(03)00273-7. [DOI] [PubMed] [Google Scholar]
- 42.Penninx BW, Kritchevsky SB, Yaffe K, et al. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol Psychiatry. 2003;54:566–572. doi: 10.1016/S0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- 43.Bindman AB, Chattopadhay A, Auerback GA. Interruptions in Medicaid coverage and risk for hospitalization for ambulatory care-sensitive conditions. Ann Intern Med. 2008;149:854–860. doi: 10.7326/0003-4819-149-12-200812160-00004. [DOI] [PubMed] [Google Scholar]
- 44.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Byles J, Young A, Furuya H, Parkinson L. A drink to healthy aging: The association between older women’s use of alcohol and their health-related quality of life. J Am Geriatr Soc. 2006;54:1341–1347. doi: 10.1111/j.1532-5415.2006.00837.x. [DOI] [PubMed] [Google Scholar]
- 46.Unützer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288:2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 47.Katon WJ, Von Korff M, Lin EH, et al. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry. 2004;61:1042–1049. doi: 10.1001/archpsyc.61.10.1042. [DOI] [PubMed] [Google Scholar]
- 48.Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006;166:2314–2321. doi: 10.1001/archinte.166.21.2314. [DOI] [PubMed] [Google Scholar]
- 49.Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363:2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Callahan CM, Boustani MA, Unverzagt FW, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295:2148–2157. doi: 10.1001/jama.295.18.2148. [DOI] [PubMed] [Google Scholar]
- 51.Coleman EA, Parry C, Chalmers S, Min SJ. The Care Transitions Intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822–1828. doi: 10.1001/archinte.166.17.1822. [DOI] [PubMed] [Google Scholar]
- 52.Brock J, Mitchell J, Irby K, et al. Association between quality improvement for care transitions in communities and rehospitalizations among Medicare beneficiaries. JAMA. 2013;309:381–391. doi: 10.1001/jama.2012.216607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 89 kb)
(DOCX 18 kb)
(DOCX 20 kb)
(DOCX 18 kb)
(DOCX 51 kb)
(DOCX 48 kb)