Figure 4.
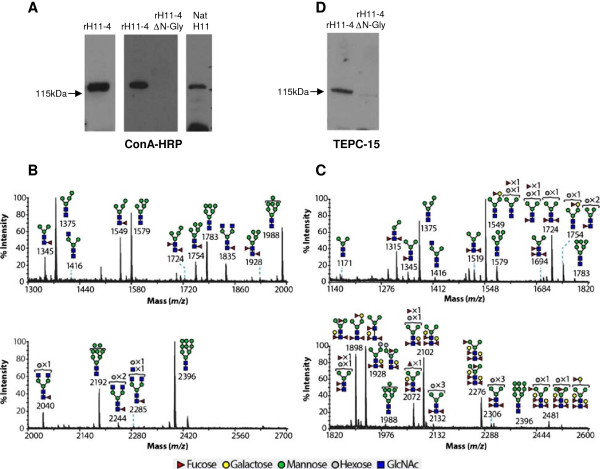
C. elegans-expressed rH11-4 protein is modified by N-linked glycosylation and phosphorylcholine. (A) Purified rH11-4 protein, rH11-4 protein in which the three potential N-glycosylation sites were mutated by site directed mutagenesis (rH11-4 ΔN-Gly) and native H11-enriched extract were separated by 4-15% SDS-PAGE, transferred to PVDF membrane and probed with HRP-labelled Con A lectin. (B and C) MALDI-TOF mass spectrometry (MS) profiles of N-linked glycans from rH11-4 protein. N-glycans of purified rH11-4 were released from tryptic glycopeptides by digestion with PNGase F (B) or PNGase A (C). Profiles of N-glycans are from the 50% MeCN fraction from a C18 Sep-Pak (Materials and Methods). All molecular ions are [M + Na]+. Putative structures are based on composition, tandem MS and biosynthetic knowledge. Structures that show sugars outside of a bracket have not been unequivocally defined. (D) Western blot of rH11-4 and rH11-4 ΔN-Gly probed with phosphorylcholine (PC) antibody TEPC-15.
