Figure 5.
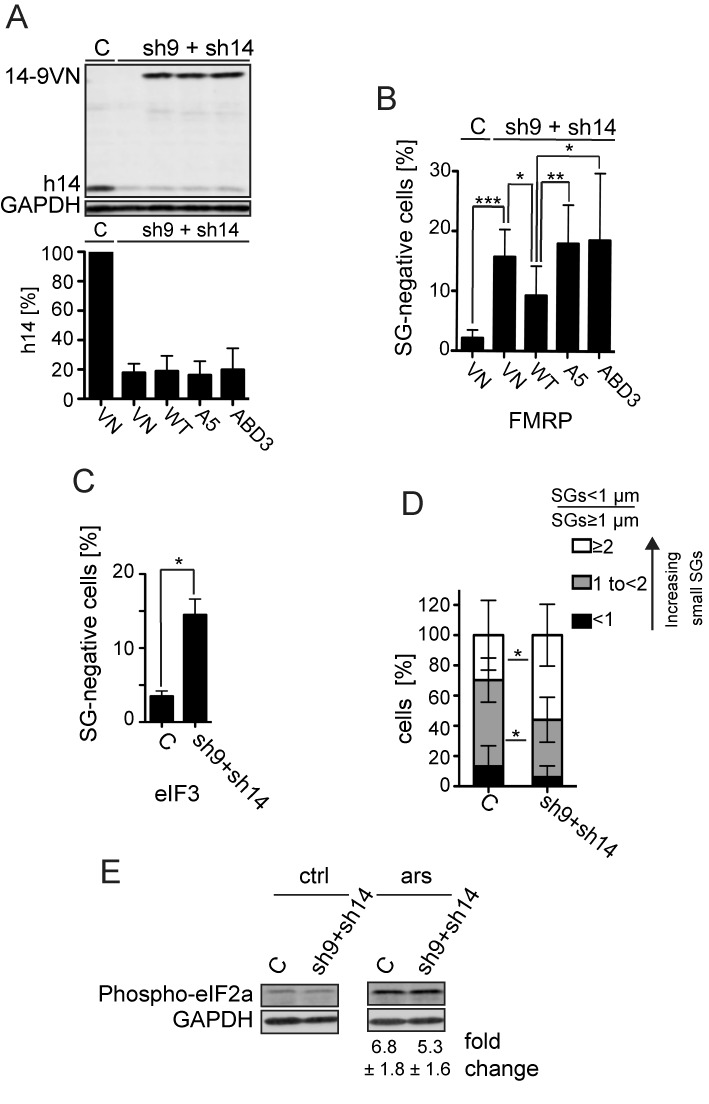
Effect of the SRP9/14 knockdown on SG formation. HeLa cells were transfected simultaneously with plasmids expressing shRNAs and the reporter proteins as indicated. After 24 h, cells were selected with puromycin (3 μg/ml) for 24 h and harvested following 30 min of sodium arsenite (500 μM) treatment at 72 h posttransfection. (A) Western blot (upper panel) and quantification standardized to GAPDH (lower panel) of the h9/14 knockdown. C: shLuc RNA; VN: venus protein; WT: 14-9VN; A5: 14-9VNA5; ABD3: 14-9VNABD3. Values were normalized to C, which was set to 100%. Error bars are shown as SD, n = 7. (B and C) Number of cells without SGs in knockdown cells. Arsenite-treated cells from (A) were subjected to immunofluorescence staining using anti-FMRP antibodies (B) or anti-eIF3 antibodies (C) and the number of cells devoid of SGs was counted in a sample of 100 cells. Error bars are shown as SD, n = 7 (B) or n = 2 (C). Unpaired one-tailed t-test. (D) Cells were categorized into three groups according to the ratio = SGs < 1 μm of diameter/SGs ≥ 1 μm of diameter. The three categories <1, 1 to <2 and ≥2 represent cells with an increasing number of small SGs. Error bars are shown as SD, n = 5 (E) Phosphorylation of eIF2α in response to stress in SRP9/14 knockdown cells. Western blots of cell lysates of arsenite-treated (ars) and untreated (ctrl) cells using anti-phospho-eIF2α antibodies. Phosphorylated protein levels were standardized to GAPDH. Numbers indicate the fold increases of phosphorylated eIF2α in response to arsenite treatment. The difference was not significant between the samples. Error bars are shown as SD; n = 4. *P≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
