FIGURE 2.
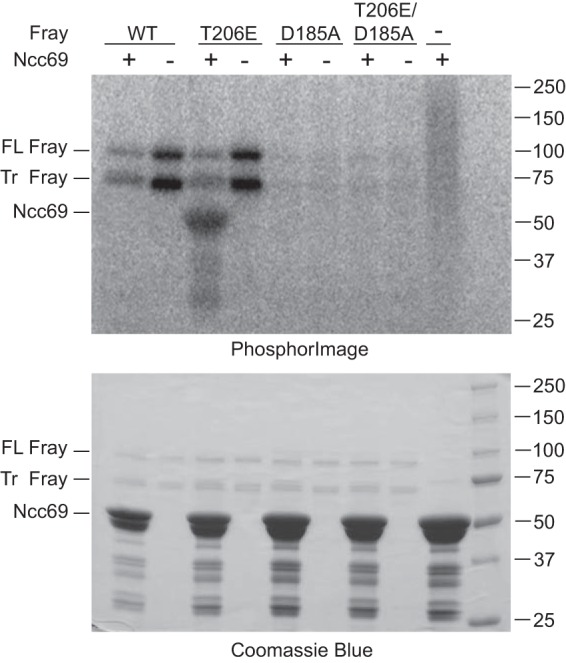
Fray phosphorylates the N terminus of Ncc69. In vitro kinase assays were performed with ∼2.5 μg of full-length GST-tagged Fray: wild-type (WT), constitutively active (T206E), kinase-dead (D185A), or constitutively active/kinase-dead (D185A/T206E), in the presence or absence of ∼20 μg GST-tagged N terminus of Ncc69 (amino acids 1–204), purified from bacteria. Bacterial expression of full-length Fray yielded both a full-length (FL) and a truncated (Tr) form, both of which autophosphorylated, whereas the kinase-dead mutants did not. Ncc69 was phosphorylated only by constitutively active FrayT206E. The top panel shows the PhosphorImager image of the Coomassie-stained gel below; this is one representative example of four experiments performed.
