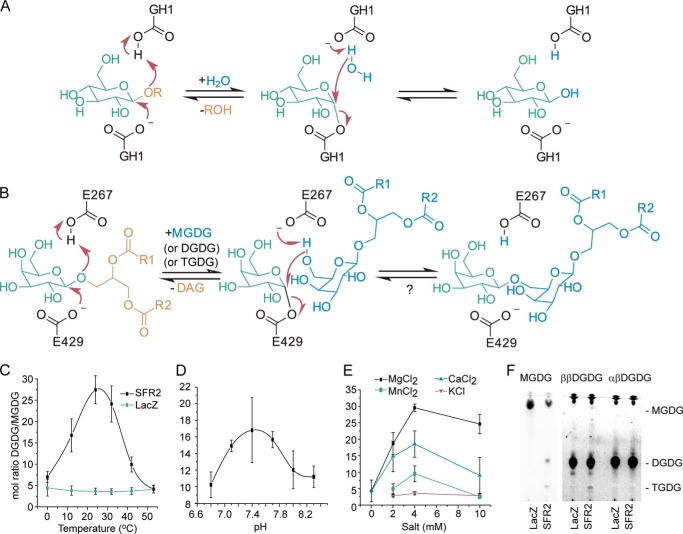
FIGURE 1.
Proposed reaction mechanism and temperature, pH, and salt dependence of SFR2 activity. A, retaining mechanism of GH1. B, expected reaction mechanism of SFR2 with residue numbers of catalytic glutamates indicated. A question mark denotes the lack of observation of the expected back reaction. R1 and R2 are aliphatic chains of 15 or 17 carbons with or without desaturation at positions 9, 11, and 15. Microsomes isolated from S. cerevisiae producing SFR2 or LacZ were incubated with MGDG under a variety of temperatures (C), biologically relevant pH values (D), or salt concentrations (E) as indicated for 30 min. Lipids were extracted, and MGDG and DGDG were separated by thin layer chromatography, converted to fatty acid methyl esters, and quantified by gas chromatography. The ratio of DGDG (a product) to MGDG (a substrate) is shown with standard deviation bars, n ≥3. F, thin layer chromatogram of assays similar to those in C–E under optimal conditions with MGDG (substrate), ββ-digalactosyldiacylglycerol (DGDG, product), or αβ-DGDG after 1 h. Chromatogram has been stained for sugars, and locations of MGDG, DGDG, and TGDG are indicated. A vertical white bar separates panels originally from the same TLC plate in which contrast settings have been increased on the right facilitate visualization of TGDG produced.