FIGURE 1.
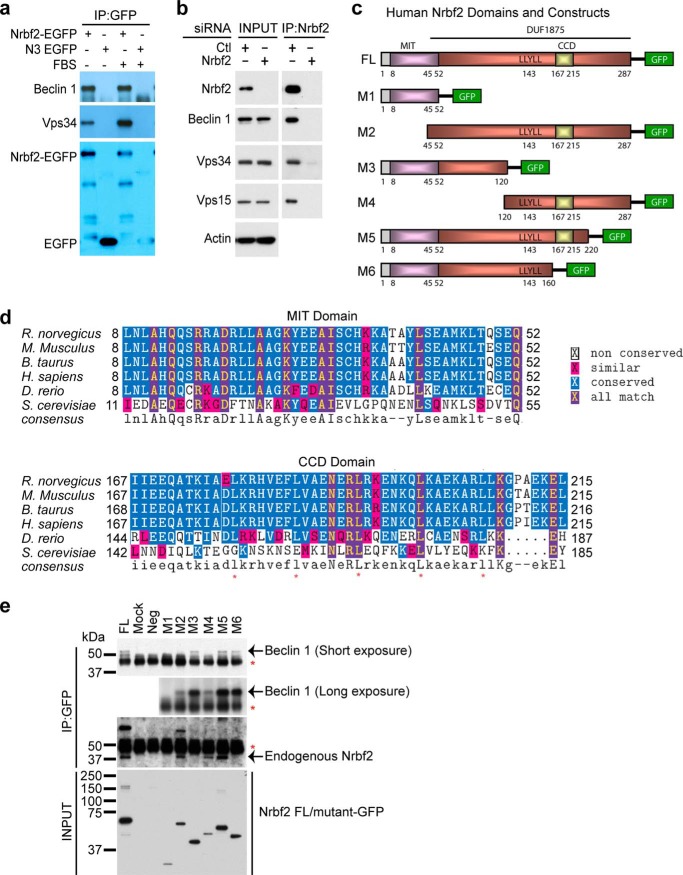
Identification of Nrbf2 as a novel Beclin 1-interacting protein. a, anti-EGFP antibody pulled down endogenous Beclin 1 and Vps34 in HEK293 cells stably expressing mouse Nrbf2-EGFP under both nutrient-rich and serum starvation conditions. Immunoprecipitated Nrbf2-EGFP and EGFP were labeled in the anti-GFP blot. b, anti-Nrbf2 antibody pulled down endogenous Beclin 1, Vps34, and Vps15 from RPE-1 cells transfected with non-targeting siRNA but not from those transfected with the SMARTpool Nrbf2 siRNA. Of note, both cell extracts before IP (INPUT) contain similar levels of Beclin 1, Vps34, Vps15, and actin. c, human Nrbf2 domain structures and diagrams of the C-terminal Cycle3 GFP-tagged full-length (FL) human Nrbf2 and truncation mutants (M1-M6) of Nrbf2. d, sequence alignment of the Nrbf2 MIT and CCD domains from different species. The five conserved leucines in the CCD are marked by red asterisks. Note that Atg38, the yeast ortholog of Nrbf2, has low sequence homology to the mammalian Nrbf2, particularly lacking the abovementioned five leucines in the CCD. e, anti-GFP IP of Cycle3 GFP-tagged full-length and mutant human Nrbf2 constructs from HepG2 cells revealed that the N-terminal 120 residues of Nrbf2 were required and sufficient for the Nrbf2-Beclin 1 interaction. Red asterisks mark the IgG bands. GFP-tagged full-length and mutant Nrbf2 constructs in the INPUT were probed with anti-GFP antibody.