FIGURE 3.
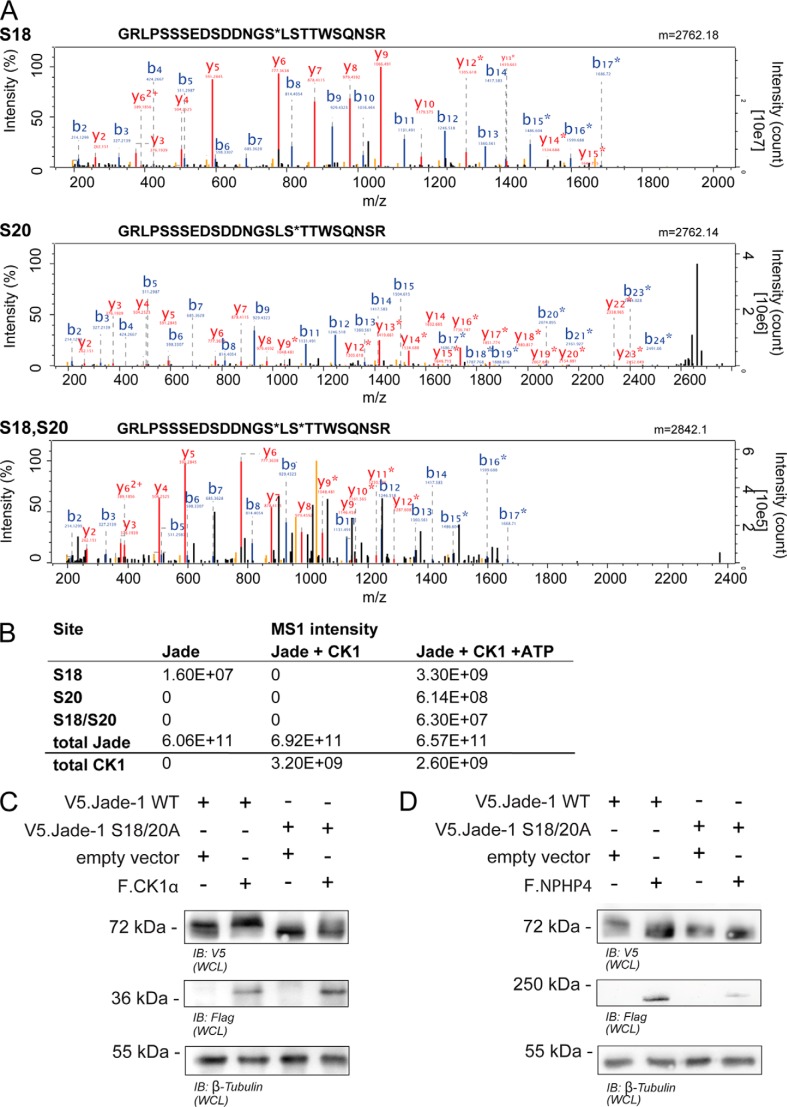
CK1α-mediated phosphorylation of Jade-1 at the SLS site is confirmed in mass spectrometry and protein expression. A, MS2 spectra of phosphopeptides identified by HCD fragmentation. Recombinant peptides were incubated with CK1 and ATP in vitro and subjected to phosphoproteomic analysis. Three phosphopeptides (containing phosphorylated Ser-18, phosphorylated Ser-20, and both Ser-18 and Ser-20) were identified with high confidence. B and y ions are annotated, and asterisks indicate phosphorylated ion species. B, intensities obtained by label-free quantification of identified Jade species. Intensities were highly increased in the presence of both CK1 and ATP. Phosphorylated species were not detected in the presence of CK1 without ATP. C and D, wild-type V5.Jade-1 or a version containing a mutated SLS motif was overexpressed in HEK 293T cells for 24 h with either F.CK1α (C) or F.NPHP4 (D) and harvested as a whole cell lysate (WCL). Mutation of the SLS site abrogated the ability of coexpressed F.CK1α to increase the expression size of V5.Jade-1, whereas the expression size of the V5.Jade-1 SLS → ALA mutant was not reduced further by coexpressed F.NPHP4. B, immunoblot.