FIGURE 1.
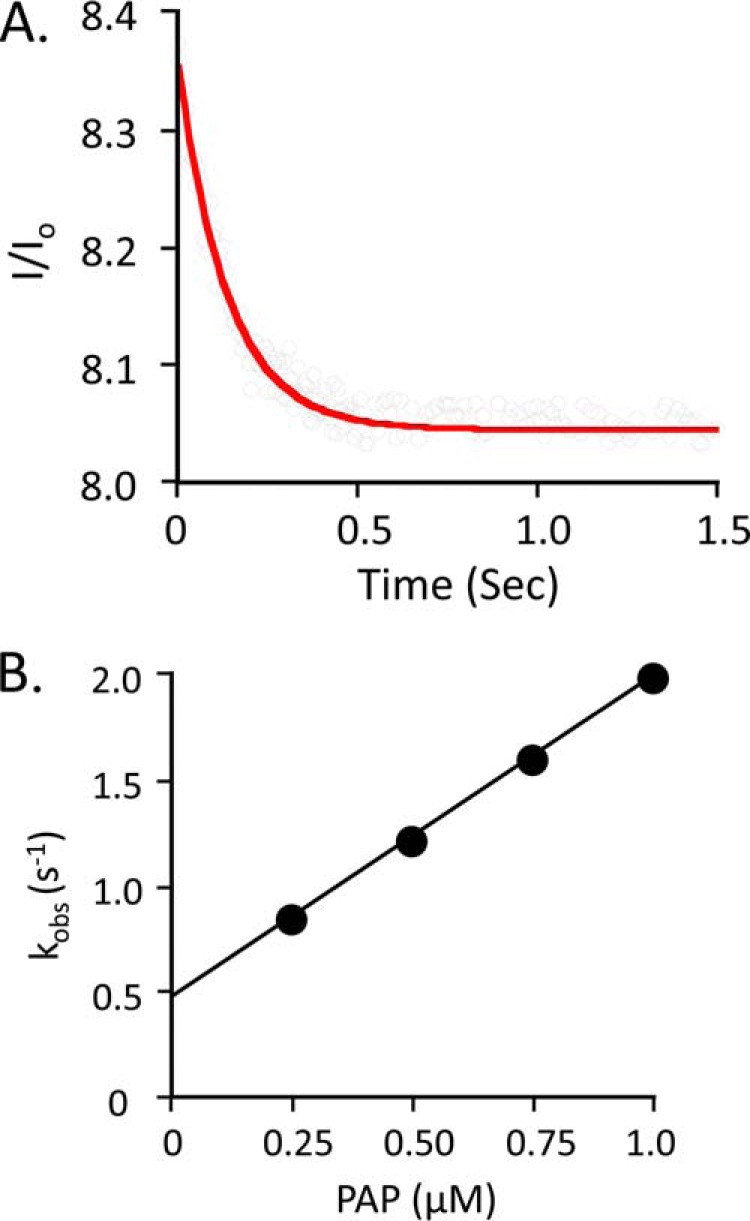
Pre-steady state binding of PAP to the SULT2A1·DHEA complex. A, the binding of PAP to SULT2A1·DHEA. Reactions were initiated by rapidly mixing (1:1 v/v) a solution containing PAP (0.25 μm, 1.0 × Kd [E·DHEA]) and DHEA (25 μm, 23 × Kd [E]) with a solution containing SULT2A1 (0.050 μm) and DHEA (25 μm). Binding was monitored by following changes in SULT2A fluorescence (λex = 290 nm, and λem ≥ 330 nm, where ex represents excitation and em represents emission). Fluorescence intensity is plotted relative to the intensity at time 0 (I/Io). Solutions contained MgCl2 (5.0 mm), NaPO4 (25 mm), pH 7.4, and were equilibrated at 25 ± 2 °C prior to mixing. The solid curve represents the best-fit behavior predicted by a single-exponential model. B, kobs versus [PAP]. Reactions were pseudo-first order in PAP in all cases. Each point represents the average of three independent determinations. The solid line through the points represents a linear least square fit of the data. The slope and intercept of the line provide kon and koff, respectively.
