Figure 1.
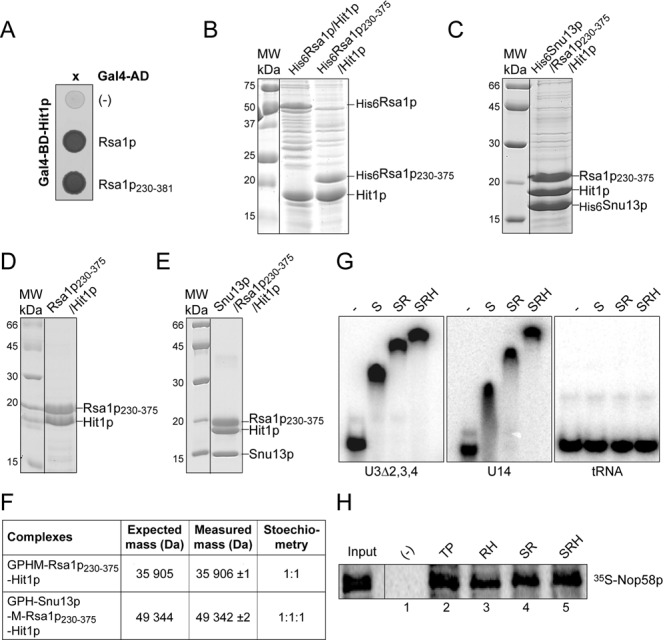
Rsa1p and Hit1p form a stable heterodimer able to bind Snu13p and Nop58p. (A) Y2H assay. Rsa1p and Rsa1p230−381 fused to the Gal4 activation domain (AD) interact with Hit1p fused to the Gal4 DNA binding domain (BD) as evidenced by growth on a His deprived medium. (B, C,D and E) Co-expression assays in E. coli and complex purifications. In (B) His6-taged Rsa1p and Rsa1p230−375 were co-expressed with Hit1p, in (C) His6-taged Snu13p was co-expressed with Rsa1p230−375 and Hit1p. Complexes were selected from crude extracts by Immobilized Metal Ion Affinity Chromatography (IMAC), followed by SDS-PAGE and Coomassie Blue staining. Molecular weight markers (MW) (in kDa) were loaded on the left. (D and E) complexes released by cleavage of the His6 tag with the PreScission protease were purified by size exclusion chromatography (Superdex 200 prep grade column, GE Healthcare) using GF buffer (10 mM HEPES pH 7.5, 150 mM NaCl, and 5 mM β-mercaptoethanol). (F) Native MS characterization of the complexes purified by gel filtration in (D) and (E). NanoESI mass spectra performed under nondenaturing conditions as described in Materials and Methods confirmed the presence of a 1:1 Rsa1p230−375−Hit1p and a 1:1:1 Snu13p−Rsa1p230−375−Hit1p complex. Removal of the His6 tag generates the N-terminal sequences GPHM for Rsa1p and GPH for Snu13p. (G) The trimeric complex His6Snu13p–Rsa1p230−375–Hit1p interacts in vitro with RNAs containing a K-turn RNA motif. Formation of RNA–protein complexes was assessed by EMSA. Radiolabeled U3Δ2,3,4 and U14 RNAs and a control tRNA were incubated with His6Snu13p (lane S), His6Snu13p–Rsa1p230−375 (lane SR), or His6Snu13p–Rsa1p230−375–Hit1p (lane SRH) complexes obtained by IMAC followed by elution in presence of 200 mM imidazole and purified as in (D) and (E). The RNP complexes formed were resolved on a 6% non-denaturing polyacrylamide gels. (H) His-pull down assays with Nop58p. Complexes formed between 35S Nop58p translated in vitro in bacterial S30 lysate and purified recombinant His6Tah1p–Pih1p (lane TP), His6Rsa1p230−375–Hit1p (lane RH), and SR and SRH complexes of panel (G) were bound to cobalt-Sepharose beads and analyzed by SDS-PAGE. 10% of the 35S Nop58p used per assay was loaded in the Input lane.
