Figure 2.
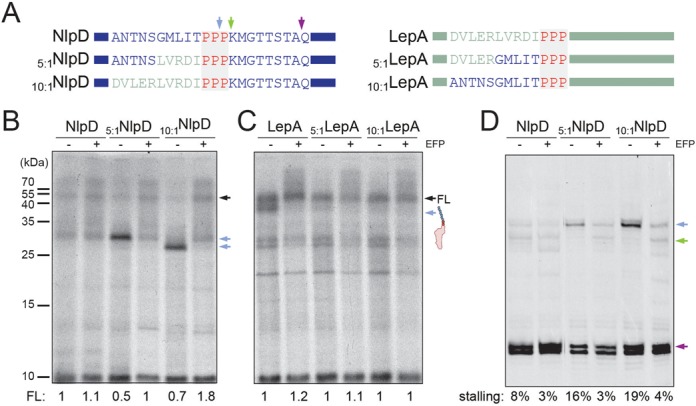
The influence of the PPP-context on efficiency of the stalling. (A) Schematic representations of chimeras constructed between NlpD and LepA with sites of stalling observed in the absence of EF-P (green and blue arrows) or in the absence of the amino acid glutamine (purple arrow). (B,C) Autoradiograms of SDS-PAGE of in vitro translation reactions of (B) wild-type NlpD, 5:1NlpD and 10:1NlpD chimeras and (C) wild-type LepA, 5:1LepA and 10:1LepA chimeras. The position of the FL protein and peptidyl-tRNA is indicated with black and blue arrows, respectively. In (B) and (C), the normalized values for the FL product are indicated for each respective lane with wild-type FL product for NlpD and LepA assigned as 1. (D) Toeprinting analyses of ribosome stalling during translation of NlpD chimeras. Stalling at peptidyl-Pro55-Pro56-tRNA in the P-site and Pro57-tRNA in the A-site (blue arrow) and with pep-PPP-tRNA in the P-site and Lys58-tRNA in the A-site (green arrow) is also shown in (A). The lack of glutamine arrests ribosomes at Q66 (violet arrow). Stalling efficiency is calculated as a percentage of stalling at PP/P and PPP/K relative to summed up intensities of PP/P, PPP/K and Q. All reactions were performed in the absence (−) and presence (+) of active EF-P.
