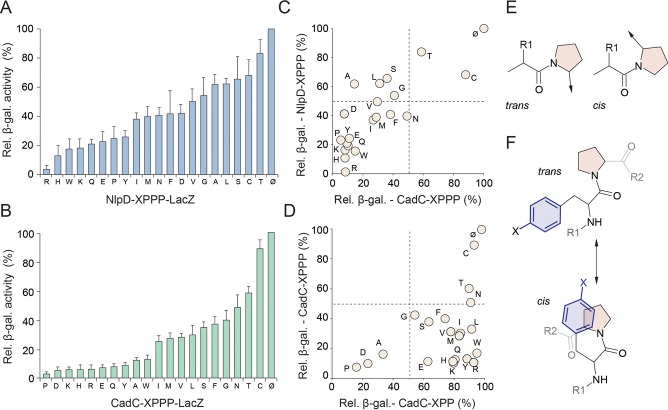
Figure 4.
Differential influence of amino acids at position -1 on efficiency of PPP-stalling. (A,B) Relative β-galactosidase activities of (A) NlpD-XPPP and (B) CadC-XPPP fusions with LacZ, where X represents one of the 20 proteinogenic amino acids, were determined by monitoring the β-galactosidase activities of the LacZ fusions in wild-type E. coli strains relative to the Δefp strain. Values were normalized relative to a control LacZ construct lacking the PPP motif (no stalling) which was assigned as 100%. (C) Scatter plot of relative β-galactosidase activities of NlpD-XPPP-LacZ relative to CadC-XPPP-LacZ constructs. (D) Scatter plot of relative β-galactosidase activities of CadC-XPPP-LacZ relative to CadC-XPP-LacZ fusions (8). (E) Chemical structure of proline in trans and cis conformation. (F) Possible trans and cis conformations of peptide containing aromatic amino acids followed by proline.