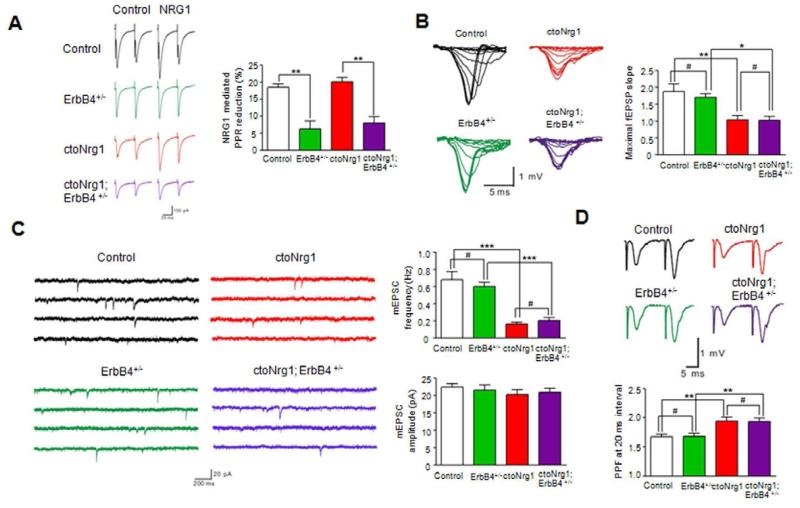
Figure 4. No effect of ErbB4 mutation on glutamatergic impairment in ctoNrg1 mice.
(A) ErbB4 heterozygous mutation attenuated NRG1-mediated PPR reduction in both control and ctoNrg1 hippocampal slices. Slices were subjected to a pair of stimulation in the absence or presence of NRG1. Left, representative eIPSC traces in ctoNrg1 and ctoNrg1;ErbB4 +/- slices; right, quantitative data. n = 10 cells from 3 mice for each group. ** p < 0.01, one-way ANOVA.
(B) No effect of ErbB4 heterozygous mutation on maximal fEPSP slope in ctoNrg1 hippocampal slices. Left, representative I/O curves in control, ErbB4 +/-, ctoNrg1 and ctoNrg1;ErbB4 +/- slices; right, quantitative data. n = 12 slices from 3 mice for each group. ** p < 0.01, * p < 0.05, # p > 0.05, one-way ANOVA.
(C) Reduced mEPSC frequency in ctoNrg1 CA1 pyramidal neurons was not altered by ErbB4 heterozygous mutation. Left, representative mEPSC traces in control, ErbB4 +/-, ctoNrg1 and ctoNrg1; ErbB4 +/- slices; right, quantitative data. n = 10 cells from 3 mice for each group. *** p < 0.001, # p > 0.05, one-way ANOVA.
(D) ErbB4 heterozygous mutation had no effect on elevated PPF at ctoNrg1 SC-CA1 synapses. Top, representative traces in control, ErbB4 +/-, ctoNrg1 and ctoNrg1; ErbB4 +/- slices; right, quantitative data at 20 ms interval, n = 12 slices from 3 mice for each group. ** p < 0.01, # p > 0.05, one-way ANOVA.