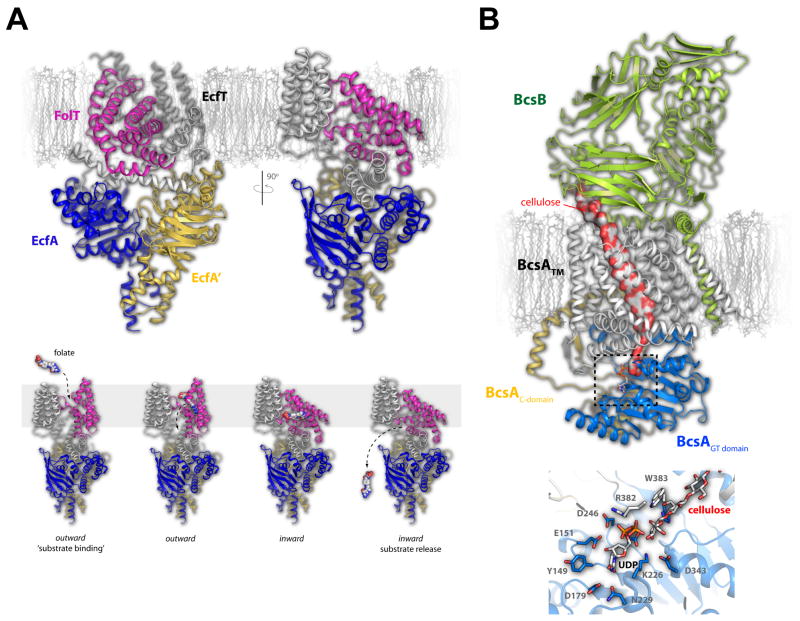
Figure 4. Folate transport by the ECF transporter and the structure of the cellulose synthase/transporter complex.
A. The fully assembled folate ECF transporter is shown in the inward facing conformation with the FolT (magenta), EcfT (gray), EcfA (blue), and EcfA’ (gold) subunits show in cartoon (top). The substrate-specific subunit, FolT, was found nearly parallel with the membrane masking the substrate binding site, leading to the proposed mechanism where this subunit must undergo a dramatic conformational shift to allow folate to bind and be transported across the membrane (bottom). B. The cellulose transporter is shown here with BcsA in gray (transmembrane domain), blue (glycosyltransferase domain), and gold (C-terminal domain) and the BcsB subunit in green. Cellulose is shown as a continuous surface (red/gray) while UDP is shown in stick. The dashed box indicates the region depicted at the bottom which is the site of glycosyltransferase activity and cellulose formation. Cellulose, UDP, and the interacting residues are shown in stick.