Fig. 1. Diagram of experimental protocol.
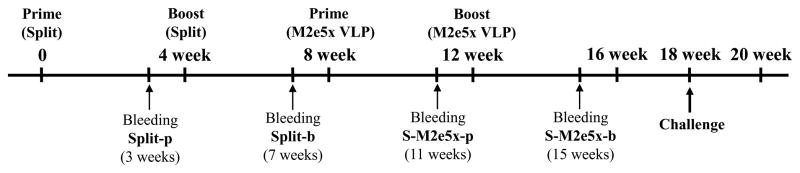
BALB/c mice (N=24) were prime and boost immunized with commercial split vaccine for human use with a 4-week interval. At 4 weeks after split boost immunization, a half of these mice were prime and boost immunized with M2e5x VLP (S-M2e5x, N=12). Sera were collected for antibody responses 3 weeks after each vaccination. In 6 weeks after M2e5x VLP boost immunization, mice were challenged with influenza A viruses, A/Philippines/2/82(H3N2) or A/Vietnam/1203/2004(rgH5N1).
